|
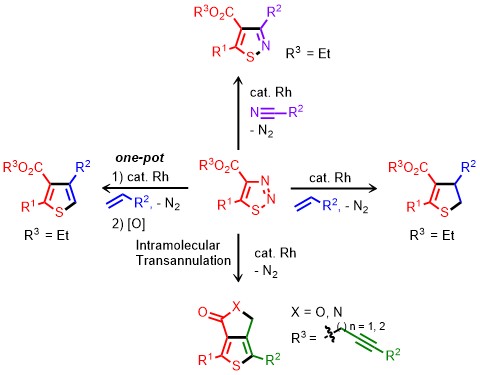
Sulfur-containing five-membered heterocyclic compounds such as dihydrothiophenes and thiophenes represent key structural motifs due to their biological activities in natural products and pharmaceuticals. In addition, thiophene derivatives are very attractive compounds in the field of material science due to their peculiar structural rigidity and useful electronic properties. Thus, the development of synthetic methods for these core scaffolds has received considerable attention in contemporary chemistry. The regioselective introduction of a wide range of substituents onto dihydrothiophene and thiophene rings from readily available starting materials is required. In this study, the regioselective synthesis of a wide range of dihydrothiophenes was developed from the rhodium-catalyzed transannulation of 1,2,3-thiadiazoles with aliphatic, aromatic, and heteroaromatic alkenes. Tandem rhodium-catalyzed transannulation of 1,2,3-thiadiazoles with alkenes followed by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) oxidation was also demonstrated for the one-pot regioselective synthesis of various thiophenes. This method was employed to efficiently synthesize pentaoligomeric compounds consisting of three benzene and two dihydrothiophene rings. Advantages of the present method include a broad substrate scope, wide functional group compatibility, and high regioselectivity. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2011-0018355), 2015H1C1A1035955. |
|
 119th General Meeting of the KCS
119th General Meeting of the KCS
 119th General Meeting of the KCS
119th General Meeting of the KCS