|
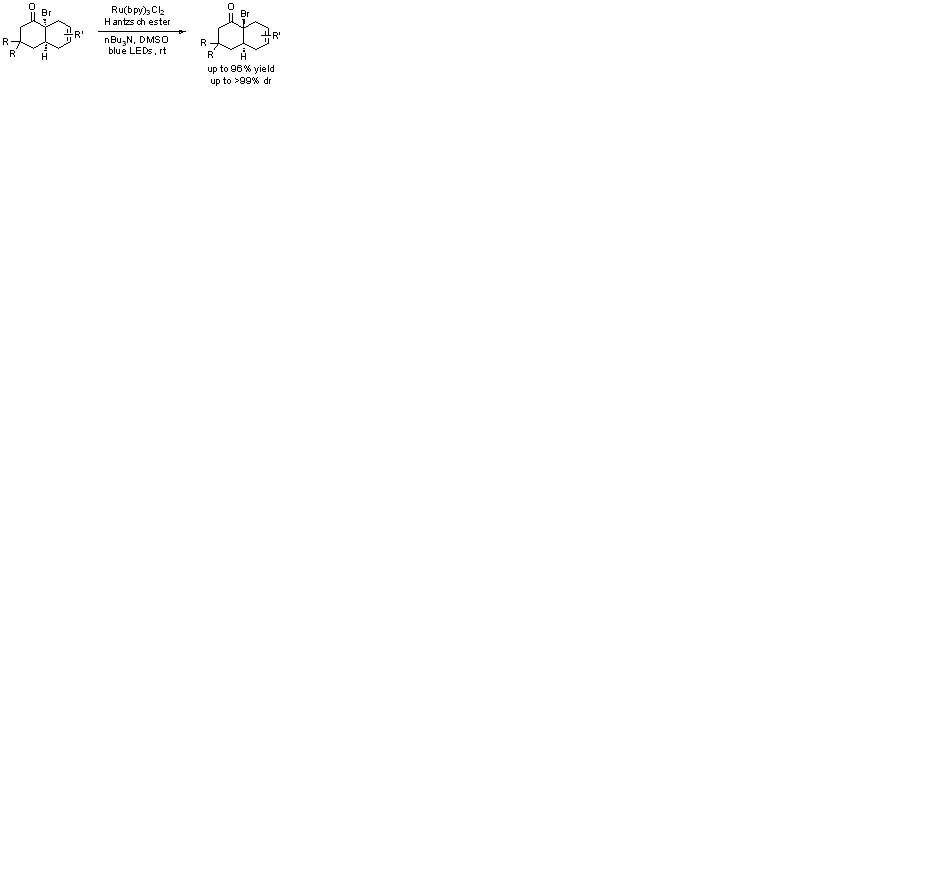
Stereoselective construction of trans-fused bicyclic systems is an important task in organic synthesis, because they are among the most versatile intermediates for the synthesis of various polycyclic natural products and relevant compounds. Danishefsky and co-workers developed a two-step trans-Diels− Alder (DA) protocol, which provides expeditious access to trans-fused octalins and hydrindanes from nitroalkene dienophiles and simple dienes. This conceptually novel strategy involves equipping cyclohexenes with a nitro group as a traceless activating group, thereby enhancing the dienophilicity of the otherwise unreactive dienophiles to yield cis-fused DA adducts. Subsequent free radical-mediated reduction of this angular functionality using AIBN and tributyltin hydride furnishes the target trans-fused bicyclic systems with good to excellent selectivity. The research groups of Danishefsky and Yamamoto have independently reported that the Lewis acid catalyzed DA reactions of α-halogentated 2-cycloalkenones with 1,3-butadienes can provide efficient access to cis-fused bicyclic systems with a halogenated quaternary stereogenic center. The halogenated cis-fused cycloadducts were then successfully converted to the trans-fused octalones in a highly stereoselective fashion by tin-mediated free radical reduction and reductive alkylation. Despite these advances, there still exists a need for developing new and green methods for the stereoselective synthesis of trans-fused polycyclic systems, because organotin hydride-mediated radical reactions have two major disadvantages: the intrinsic toxicity of tin-based reagents and the difficulty of removing their residues from the products. We envisaged that visible-light-induced photocatalytic dehalogenation might be an ideal platform for the development of a green and environmentally benign trans-DA protocol that is free of organotin reagent. This presentation will deal with the full details of this novel reaction. |
|
 119th General Meeting of the KCS
119th General Meeting of the KCS
 119th General Meeting of the KCS
119th General Meeting of the KCS