|

|
| Type |
Symposium |
| Area |
[SRC 심포지엄] New Trends and Developments in Organic Synthesis |
| Room No. |
303호 |
| Time |
WED 16:40-: |
| Code |
KCS5-12 |
| Subject |
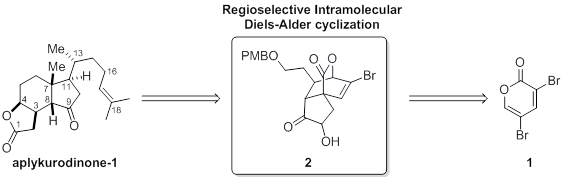
Total synthesis of aplykurodinone-1 by Efficient Regio-selective Intramolecular Diels-Alder of 2-pyrone |
| Authors |
이준호, 조천규1,*
한양대학교 자연과학 화학과, Korea
1한양대학교 화학과, Korea
|
| Abstract |
|
Aplykurodines are steroids natural products which have tricyclic ring core. Many aplykurodines show cytotoxic activities against a range of human cancer cell lines . Natural product aplykurodinone-1 which we eager to synthesize have a unsaturated side chain linked to the unusual cis-fused hydrindane moiety with six contiguous stereocenters.
As a part of our ongoing study on 3,5-dibromo-2-pyrone toward target oriented synthesis, we devised a new synthetic route to aplykurodinone-1 by way of tricyclic lactone that we envisioned to access with an intramolecular Diels-Alder reaction of 2-pyrone containing vinyl ketone group as dienophile. The IMDA reaction indeed allowed us to the key tricyclic lactone intermediate. Subsequent reactions including lactone ring opening will culminate in the total synthesis of the titled natural product.
|
|
|
|
| E-mail |
ionahwow@hanyang.ac.kr |
|
 119th General Meeting of the KCS
119th General Meeting of the KCS
 119th General Meeting of the KCS
119th General Meeting of the KCS