|
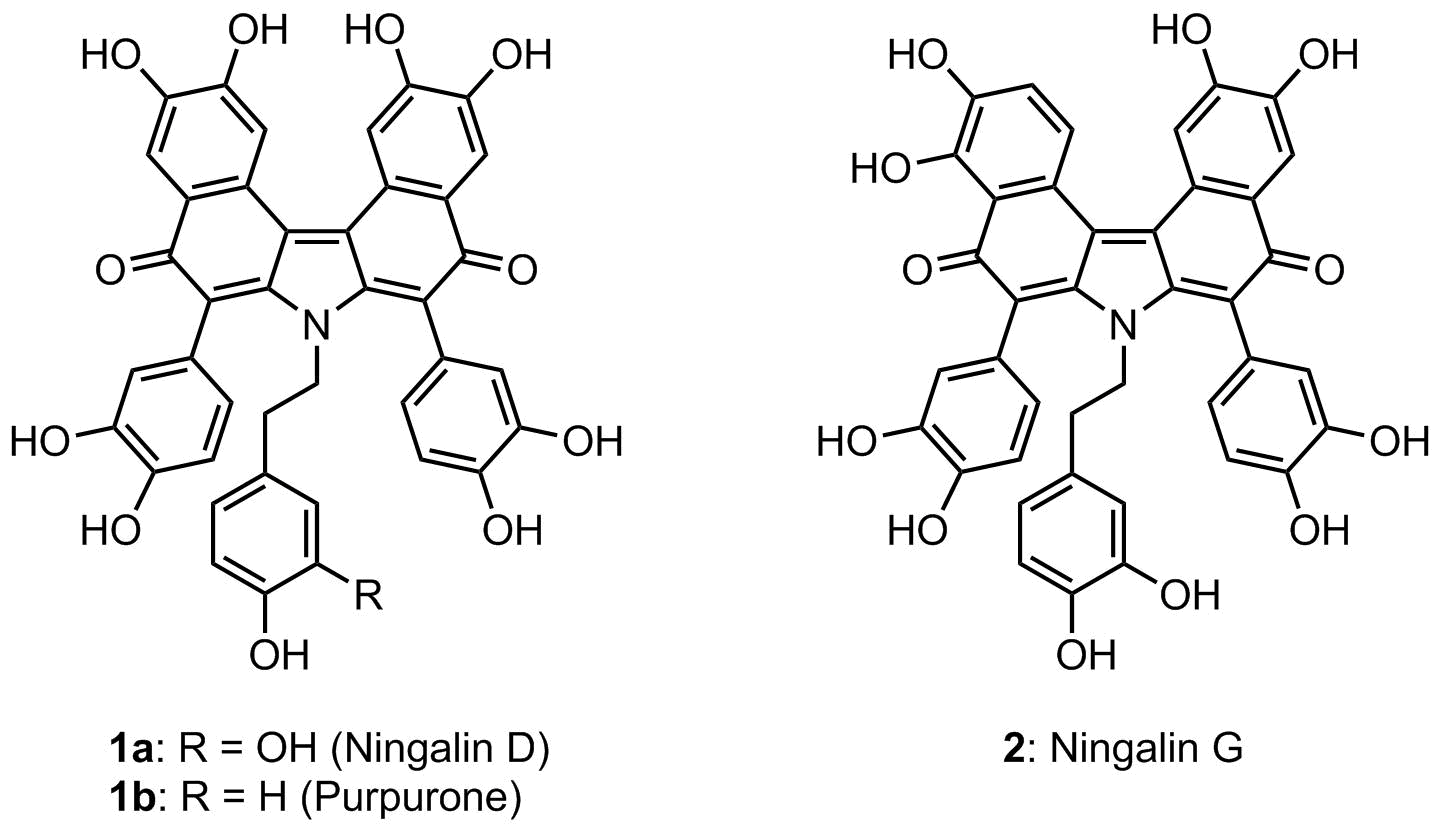
Ningalin D is a purple colored benzocarbazole marine alkaloid first isolated by Kang et al. from a western Australian unidentified ascidian belonging to the genus Didemnum. More recently, Capon et al. isolated a closely related marine natural product, named as ningalin G, from the extract of a Southern Australian marine ascidian, Didemnum, and characterized its structure as shown below (Figure 1). As a part of our ongoing investigation on the synthetic utility of aryl hydrazides, we have elaborated a new synthetic route to both ningalin D (1a) and G (2), by way of 7H-dibenzo[c,g]carbazole as the key juncture. Subsequent installation of 3,4-dimethoxyphenyl groups followed by a series of oxidation reactions permitted the total syntheses of the titled marine alkaloids in good overall yields. |
|
 119th General Meeting of the KCS
119th General Meeting of the KCS
 119th General Meeting of the KCS
119th General Meeting of the KCS