|
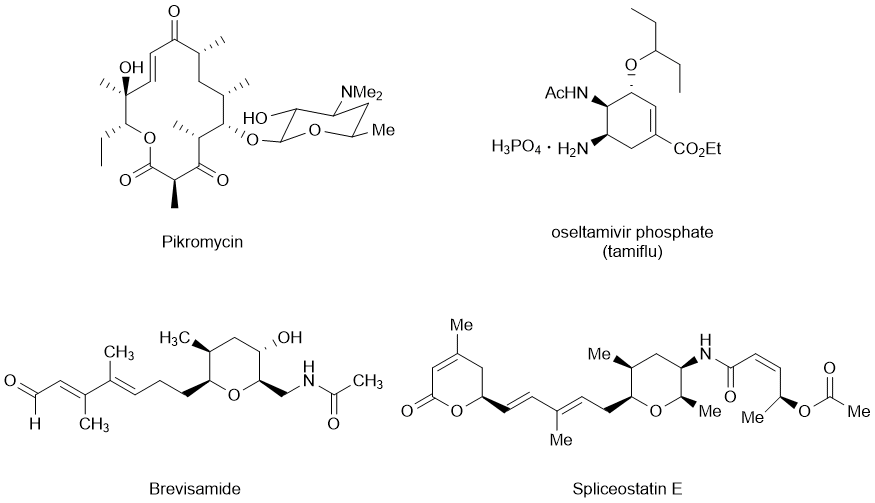
Cyclization is one of the most important types of reactions for the synthesis of cyclic natural products. Macrolide antibiotics are a group of compounds that contains a macrolactone ring to which one or more sugars are attached. Pikromycin is the first isolated macrolide antibiotics before the advent of erythromycin, one of the most renowned macrolide antibiotics. We have exploited successfully the ring-closing metathesis (RCM) reaction for cyclization to secure the framework of this macrolide. Oseltamivir Phosphate (Tamiflu), one of the most typical inhibitors for neuraminidase, contains a six-membered carbocycle. We have also adopted the RCM strategy for the synthesis of oseltamivir phosphate, and have discovered that cis-2,3-bis(hydroxymethyl)aziridine is a good starting material for the synthesis of oseltamivir as well as other nitrogen-containing natural products via ring-opening reactions. The RCM based strategy has also been applied to the stereoselective synthesis of (−)-brevisamide, a monocylic ether amide, containing a characteristic tetrahydropyran ring with an array of substituents. In order to develop more general cyclization strategy for the tetrahydropyran-containing natural products we have investigated the cyclization methods based on the ring opening of epoxides and iodoetherification aiming the synthesis of brevisamide as well as spliceostatin E. |
|
 119th General Meeting of the KCS
119th General Meeting of the KCS
 119th General Meeting of the KCS
119th General Meeting of the KCS