|
Transition-metal-catalyzed carbonylation using carbon monoxide is one of the significant methods to prepare a variety of carbonyl compounds. In particular, Pd-catalyzed carbonylative cross-coupling is an important method for the synthesis of a large number of compounds having the carbonyl functional group. However, the carbonylation of metal carbenes is rarely reported due to the limitations of substrate scope and harsh conditions, such as the high pressure of carbon monoxide, high reaction temperature, and stoichiometric processes. Moreover, because the carbonylation of metal carbene furnishes ketenes, which are very important in organic synthesis, the development of streamlined synthetic methods to overcome these shortcomings is still highly attractive and challenging.
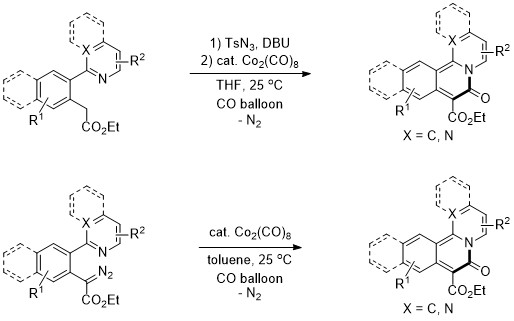
In conclusion, we have successfully developed a dicobalt octacarbonyl catalyzed carbonylative cyclization of pyridinyl diazoacetates for the synthesis of pyridoisoquinolinones under a carbon monoxide atmosphere. Moreover, a useful synthetic method for a wide range of pyridoisoquinolinones from pyridinyl aryl acetates has been demonstrated through diazotization using TsN3 and DBU followed by Co-catalyzed carbonylation and intramolecular cyclization of ketene with a tethering pyridinyl moiety under a carbon monoxide atmosphere in a semi-one-pot procedure. These transformations are attractive due to the use of an inexpensive and commercially available Co catalyst and an easily accessible starting material and the release of harmless N2 under mild conditions (room temperature).
|
|
 120th General Meeting of the KCS
120th General Meeting of the KCS
 120th General Meeting of the KCS
120th General Meeting of the KCS