|
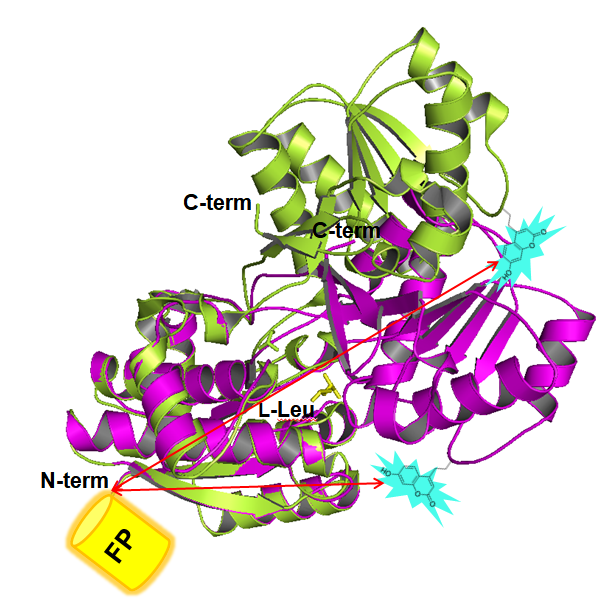
Periplasmic binding proteins (PBPs) are members of a widely distributed protein superfamily found in bacteria and archaea, and involved in cellular uptake of solute. In this report, a leucine binding PBP was engineered to detect L-Leu by FRET change upon ligand binding. A fluorescent unnatural amino acid, L-(7-hydroxycoumarin-4-yl)ethylglycine (CouA), was genetically incorporated into the protein as a FRET donor, and yellow fluorescent protein (YFP) was fused to its N-terminus as a FRET acceptor. When CouA was incorporated into 178 position, the sensor protein showed 2.5-fold increase in FRET ratio. By engineering the protein, its substrate specificity was significantly improved, showing minimal FRET ratio change with the other 19 natural amino acids and D-Leu. Further engineering made the sensor protein more sensitive (14-fold) for L-Leu, and recognize L-Met as well with moderate binding affinity. Selected mutant sensors were used to measure L-Leu concentration in a biological sample (fetal bovine serum) and optical purity of Leu and Met. This FRET-based sensor design strategy allowed us to readily engineer the natural receptor to have improved binding affinity and specificity, and to recognize other natural molecules which are not a ligand for the wild-type receptor. The design strategy can be applied to other natural receptors and would make it possible to engineer the receptors to sense biochemically more interesting molecules. |
|
 120th General Meeting of the KCS
120th General Meeting of the KCS
 120th General Meeting of the KCS
120th General Meeting of the KCS