|

|
| Type |
Poster Presentation |
| Area |
Electrochemistry |
| Room No. |
Exhibition Hall 2+3 |
| Time |
10월 20일 (금요일) 13:00~14:30 |
| Code |
ELEC.P-484 |
| Subject |
Electrodeposition of Cobalt Selenide Thin Films: A Combined Voltammetry/Electrochemical Quartz Crystal Microgravimetry Study |
| Authors |
Hyung-woo Jee, YunHyeok Jang1, KONGSHIK RHO1, Ki Jung paeng, Noseung Myung1,*
Department of Chemistry, Yonsei University, Korea
1Department of Applied Chemistry, Konkuk University, Korea
|
| Abstract |
|
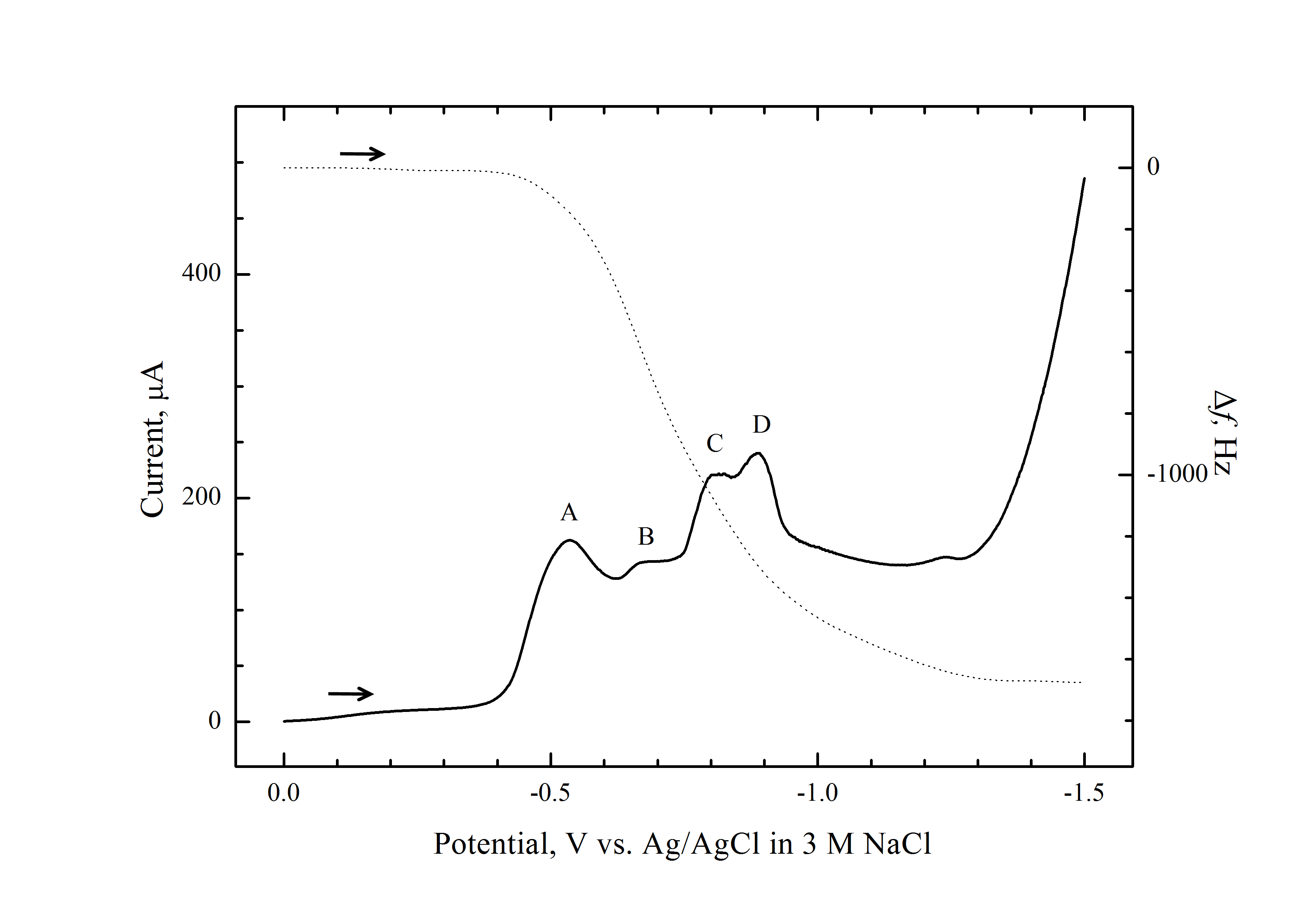
The mechanism of electrodeposition of cobalt selenide (CoSe) thin films was investigated by the combined application of linear sweep voltammetry (LSV) and electrochemical quartz crystal microgravimetry (EQCM) on Pt-coated quartz electrodes. Cobalt selenide films were electrodeposited on the Pt surface from 0.1 M Na2SO4 electrolyte solution containing 5 mM SeO2 and 5 mM Co(CH3COO)2 by linear sweep voltammetry. Four cathodic waves were observed during the linear scans and the reactions corresponding to these waves were investigated with LSV and EQCM. Combined stripping voltammetry and EQCM showed that CoSe was electrodeposited via two routes: (1) Underpotential deposition of Se followed by deposition of cobalt as CoSe; and (2) Reaction of Co(II) with electrogenerated Se(-II) to result in CoSe. Compositional analyses revealed that the electrodeposited films contained CoSe and free Se, depending on the deposition potential. However, no cobalt was found in the films due to chemical (galvanic) instability of the cobalt film in the deposition bath. |
|
|
| E-mail |
jhoabc@naver.com |
|
 120th General Meeting of the KCS
120th General Meeting of the KCS
 120th General Meeting of the KCS
120th General Meeting of the KCS