|
Proper analysis and identification of reaction intermediates in catalysis often leads to new reaction discoveries. In this presentation, I will describe some of our recent investigations in asymmetric gold catalysis as well as Brønsted acid catalysis.
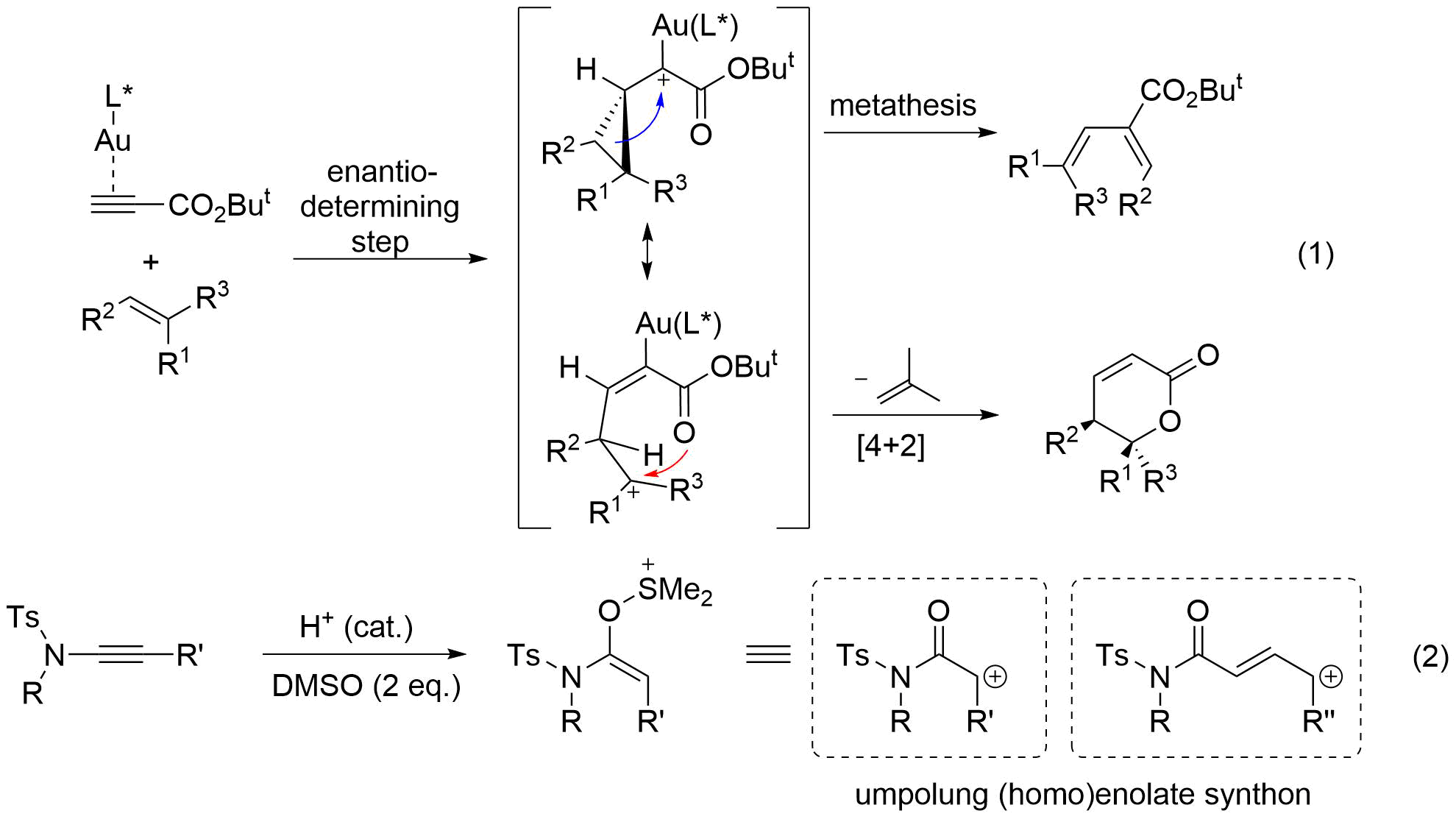
Despite remarkable advances in gold catalysis during the last decade, there is only a few intermolecular reactions, especially in an enantioselective sense.1 In the reaction between alkynes and alkenes, enantio-determining step involves discrimination of prochiral face of the olefin nucleophile, approaching away from the linearly coordinated gold complex (Eq. 1).2a After extensive efforts, we found that 1,1,2,2-tetrachloroethane and AgNTf2 as the optimal combination of solvent and counter-anion. Although 80~98%ee was achieved, some substrates gave a lower yield of [4+2] product, due to the unwanted formation of metathesis and conjugate addition product. Serendipitously, we found that addition of SDS surfactants significantly suppressed these side pathways, allowing an efficient access to diverse α,β-unsaturated-δ-lactones.2b,c
Organocatalytic activation of C-C multiple bonds are much less common than C=N or C=O activation. In this vein, ynamide derivatives are a special class of alkynes that can be activated by Brønsted acids. Recently, we have uncovered acid-catalyzed oxidation of ynamides can be achieved with pyridine-N-oxide or dimethyl sulfoxide as terminal oxidants, emulating a carbene reactivity.3 This umpolung approach allows bimolecular coupling of a wide range of nucleophiles, which is otherwise difficult to achieve.
|
|
 120th General Meeting of the KCS
120th General Meeting of the KCS
 120th General Meeting of the KCS
120th General Meeting of the KCS