|
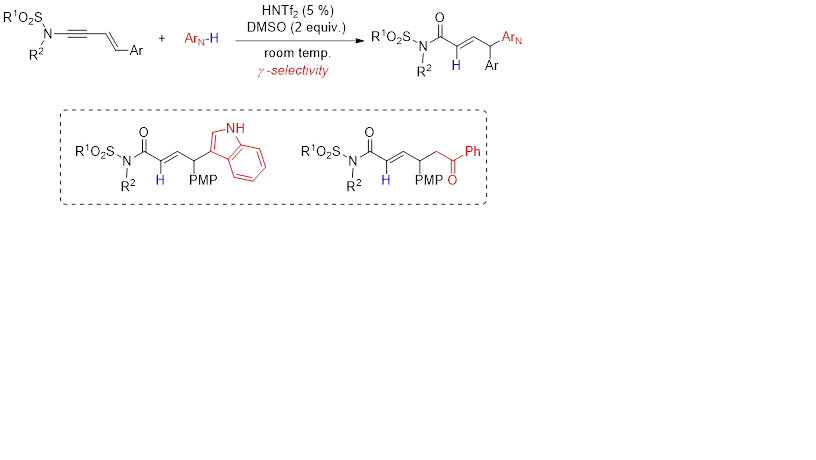
Recently, we have shown that the Brønsted acid-catalyzed reaction of ynamides displays a formal enolate umpolung reactivity, allowing substitution of various arene nucleophiles at the α-position of the carbonyls. Herein, we extended this method to enynamides and observed a good to excellent level of regioselectivity for the γ-substitution of the amides. Depending on the nucleophiles, unactivated indoles and silyl enol ethers participated as excellent nucleophiles, leading to γ-indolyl-α,β-unsaturated amides and 1,6-dicarbonyl compounds, respectively. Importantly, dimethyl sulfoxide could be used as innocuous and atom-efficient oxidants that can replace previously described pyridine-N-oxides.
|
|
 120th General Meeting of the KCS
120th General Meeting of the KCS
 120th General Meeting of the KCS
120th General Meeting of the KCS