|

|
| Type |
Oral Presentation |
| Area |
Oral Presentation in Chemistry of Life |
| Room No. |
Room 203 |
| Time |
THU 09:20-: |
| Code |
BIO.O-2 |
| Subject |
In search of a next generation Bedaquiline for the treatment of Tuberculosis
|
| Authors |
Peter Choi*, Hamish S. Sutherland, Amy S.T. Tong, Adrian Blaser, Daniel Conole, Christopher B. Cooper1, Scott G. Franzblau2, Anna M. Upton1, William A. Denny, Brian D. Palmer
Auckland Cancer Society Research Centre, University of Auckland, New Zealand
1Global Alliance for TB Drug Development, United States
2Institute for Tuberculosis Research, University of Illinois at Chicago, United States
|
| Abstract |
|
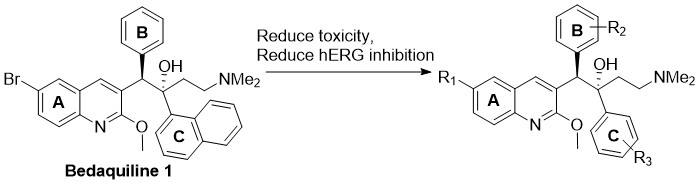
Bedaquiline (1) is the first FDA approved drug for the treatment of tuberculosis (TB) in 40 years and the first of the diarylaminoquinoline class. It has a novel mechanism of action through inhibition of the mycobacterial ATP synthase enzyme.1 It demonstrates excellent efficacy against TB, but induces phospholipidosis at high doses, has a long terminal elimination half-life (due to its high lipophilicity) and exhibits potent hERG channel inhibition, resulting in clinical QTc interval prolongation.2
A range analogues of 1 have been prepared to address these issues and evaluated for their anti- M.tb activity (MIC90) to examine their use as effective and safer second generation analogues of 1.
References
1. Koul, A.N.; Dendouga, K.; Vergauwen, B.; Molenberghs, B.; Vranckx, L.; Willebrords, R.; Ristic, Z.; Lill, H.; Dorange, I.; Guillemont, J.; Bald, D;, Andries, K. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 2007, 3, 323-324.
2. Kakkar, A.K.; Dahiya, N. Bedaquiline for the treatment of resistant tuberculosis: Promises and pitfalls. Tuberculosis 2014, 94 (4), 357-362.
|
|
|
|
| E-mail |
p.choi@auckland.ac.nz |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS