|

|
| Type |
Oral Presentation |
| Area |
Oral Presentations of Young Scholars in Organic Division |
| Room No. |
Halla Hall B |
| Time |
THU 09:45-: |
| Code |
ORGN.O-4 |
| Subject |
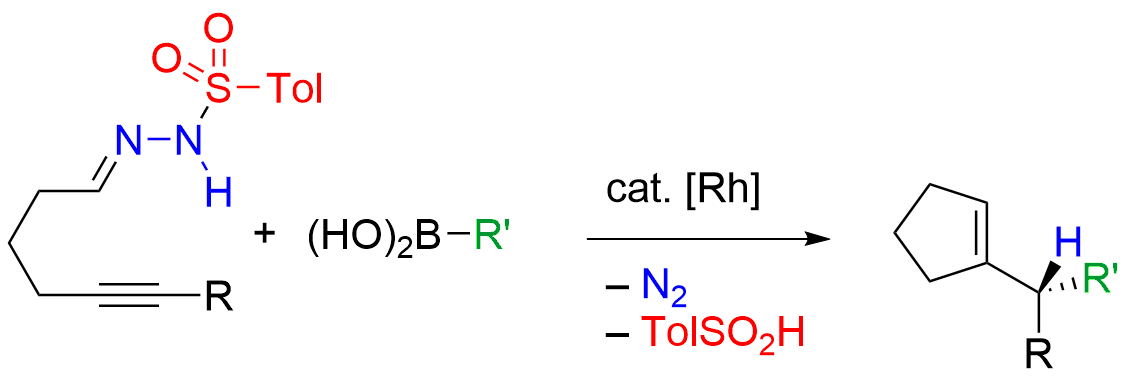
Rhodium-Catalyzed Tandem Addition―Cyclization―Rearrangement of Alkynylhydrazones with Organoboronic Acids |
| Authors |
Kyoungmin Choi, Hoyoon Park1, Chulbom Lee1,*
Department of Chemistry, Seoul National University, Korea
1Division of Chemistry, Seoul National University, Korea
|
| Abstract |
|
In this talk, a tandem protocol for the synthesis of cycloalkenes based on the merger of rhodium catalysis and traceless pericyclic rearrangement will be presented. This strategy enables alkyne-tethered N-sulfonylhydrazones and organoboronic acids to undergo a cascade of addition―cyclization―rearrangement reactions to provide cycloalkene products. Mechanistic studies suggest that the process is commenced by the rhodium-catalyzed addition―cyclization and completed with the retro-ene reaction of an allylic diazene intermediate. The novel transformation can be rendered asymmetric by using chiral diene ligands for the rhodium catalyst, whereby enantioselective addition to the C=N bond establishes the C―N stereocenter whose chirality is transferred to an allylic C―H center via suprafacial rearrangement. |
|
|
|
| E-mail |
ckm2310@snu.ac.kr |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS