|

|
| Type |
Poster Presentation |
| Area |
Organic Chemistry |
| Room No. |
Event Hall |
| Time |
4월 19일 (목요일) 11:00~12:30 |
| Code |
ORGN.P-320 |
| Subject |
Pd-Catalyzed Propargyl Substitution and Cycloisomerizatioh for the Synthesis of Multisubstituted Allenes, Furans, and Pyrroles |
| Authors |
Yoonbeak Lee, Sang Hoon Han1, Phil Ho Lee1,*
department of chemistry, Kangwon National University, Korea
1Department of Chemistry, Kangwon National University, Korea
|
| Abstract |
|
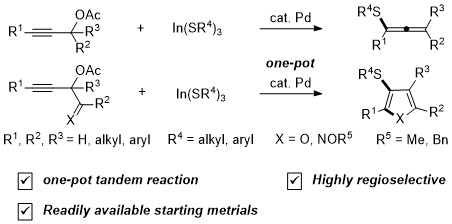
Recently, an efficient synthetic method towards multisubstituted furans and pyrroles bearing hetero-substituents was reported through metal-catalyzed 1,2-shifts of diverse migrating groups in allenyl systems. However, the introduction of a wide variety of substituents at the 4-position of furans and pyrroles is impossible due to requirement of [1,3]-H shift in these methods. Therefore, the development of an efficient synthetic method for multisubstituted furans and pyrroles bearing 3-heteroatom substituents as well as substituents at the 4-position has been a continuing challenge.
Herein, we report Pd-catalyzed propargyl substitution reactions of propargyl acetates with indium organothiolates for the synthesis of multisubstituted allenyl sulfides. This procedure employed tandem Pd-catalyzed propargyl substitution and cycloisomerization reactions from indium organothiolates and propargyl acetates bearing acyl and imidoyl groups for the synthesis of multisubstituted furans and pyrroles in one-pot.
This work was supported by the Human Resource Training Program for Regional Innovation and Creativity through the Ministry of Education and National Research Foundation of Korea (NRF-2015H1C1A1035955) |
|
|
|
| E-mail |
dbsqor0309@naver.com |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS