|
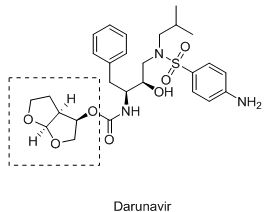
bis-Tetrahydrofuran in HIV-1 protease inhibitors is an attractive synthetic target because of its key role in showing excellent enzyme inhibitory and antiviral activity. Recently, we developed asymmetric synthesis of bis-tetrahydrofuran with high stereoselectivity and efficiency. The key step is Pd-catalyzed hydroalkoxylation followed by ring closing metathesis (RCM) and radical cyclization.1
1. a) Lim, W.; Kim, J.; Rhee, Y. H., J. Am. Chem. Soc., 2014, 136, 13618. b) Kim, M.; Kang, S.; Rhee, Y. H., Angew. Chem. Int. Ed., 2016, 55, 9733. |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS