|

|
| Type |
Poster Presentation |
| Area |
Organic Chemistry |
| Room No. |
Event Hall |
| Time |
4월 19일 (목요일) 11:00~12:30 |
| Code |
ORGN.P-379 |
| Subject |
Synthetic studies of antitumor antibiotic aminocyclopentitol natural products from Streptomyces pactum var. pactum |
| Authors |
TAEJUNG KIM, So Matsudaira1, Shohei Matsushita1, Young-Tae Park, Masaya Nakata1, Jungyeob Ham*, Yoko Saikawa1,*
Natural Products Research, Korea Institute of Science and Technology, Korea
1Department of Applied Chemistry, Keio University, Japan
|
| Abstract |
|
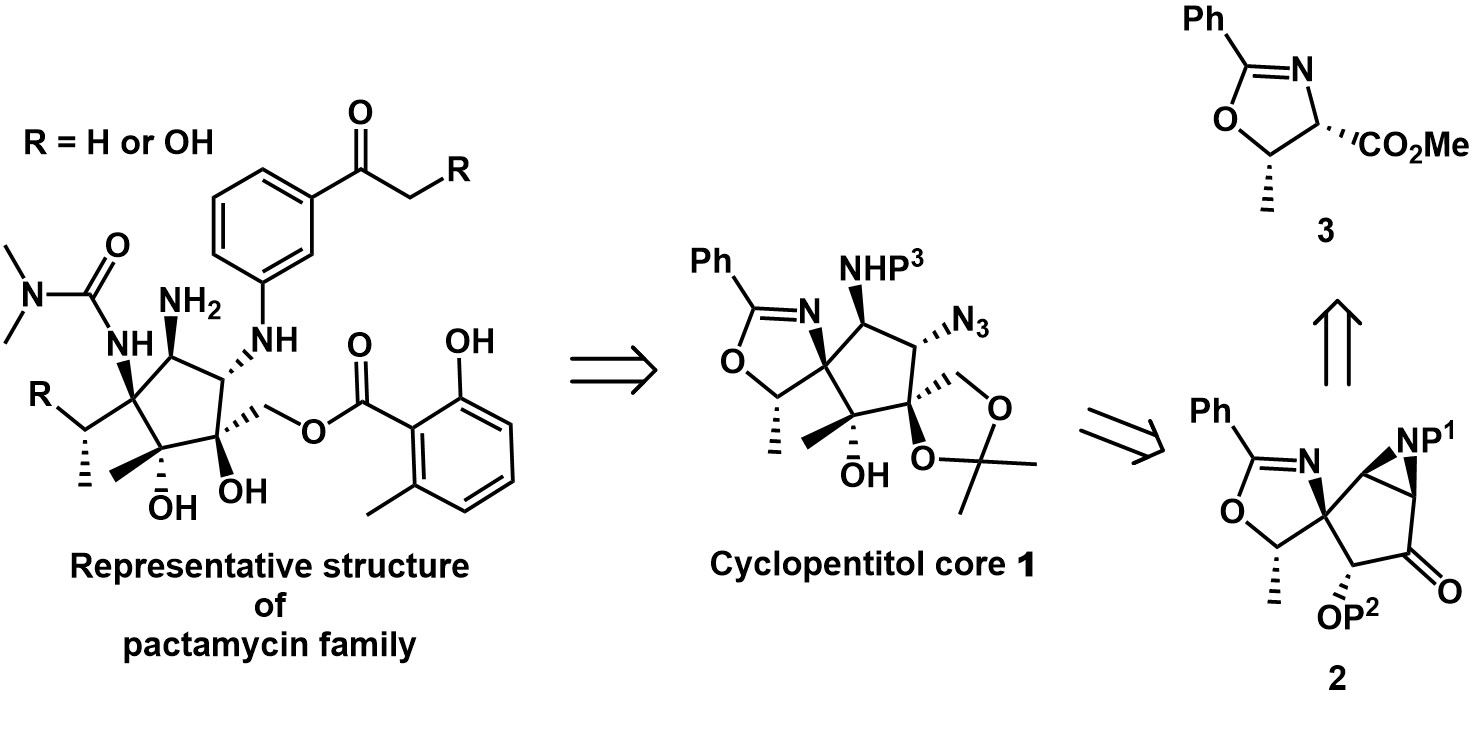
Pactamycin family of natural products, microbial secondary metabolites produced by Streptomyces pactum var. pactum, has a wide range of biological properties, including antimicrobial, antitumor, antiviral, and antiprotozoal activities, and affects the cell growth of all three phylogenetic domains, eukarya, bacteria, and archaea. They consist of a densely functionalized cyclopentitol core featuring six contiguous stereogenic centers, three of which are fully substituted. Also, two aromatic rings (meta-acetylphenyl and 2-hydroxy-6-methylbenzoyl) and urea groups adorn the core structure.
As part of the project directed toward the synthesis of cyclopentitol core 1, we designed aziridine 2, which offers a convenient five-carbon ring skeleton; every center of the ring can be potentially functionalized. The construction of the carbon framework of these natural products is started with L-threonine-derived oxazoline methyl ester 3, which was converted to aziridine 2 in 12 steps, including ring-closing metathesis (RCM) and stereoselective aziridine formation. Reproducible chemical methods enabling the synthesis of cyclopentitol core 1 containing an octa-substituted cyclopentane have been developed. Further studies toward the total synthesis of pactamycin family of natural products from 1 are now in progress and the results of oxazoline ring-opening reaction followed by construction of the urea moiety and the meta-acetylphenyl group introduction by Cu-catalyzed C-N bond formation reaction will be disclosed in due course. |
|
|
| E-mail |
kgsing@gmail.com |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS