|

|
| Type |
Poster Presentation |
| Area |
Organic Chemistry |
| Room No. |
Event Hall |
| Time |
4월 19일 (목요일) 11:00~12:30 |
| Code |
ORGN.P-386 |
| Subject |
Selective Ring-Opening of N‑Alkyl Pyrrolidines with Chloroformates to 4‑Chlorobutyl Carbamates |
| Authors |
ChungHyeon Yu, Hyun-Joon Ha1, Eun Jin Cho2,*
Department of Bionanotechnology, Hanyang University, Korea
1Department of Chemistry, Hankuk University of Foreign Studies, Korea
2Department of Chemistry, Chung-Ang University, Korea
|
| Abstract |
|
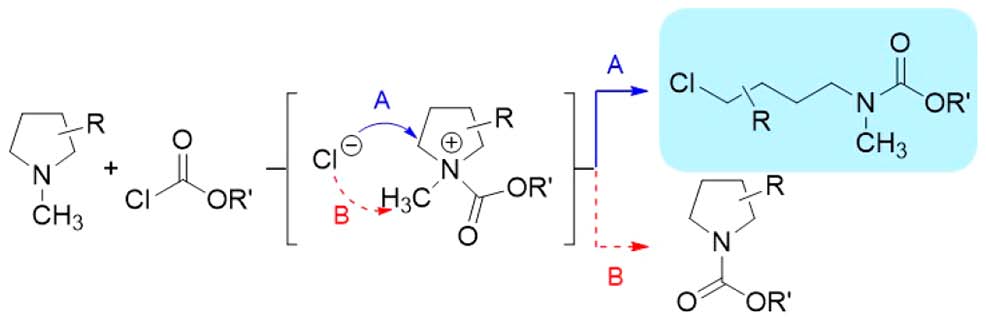
Our study shows that among aza-heterocycles of various ring sizes, including aziridines, azetidines, pyrrolidines, and piperidines, only N-alkyl pyrrolidines undergo competitive reaction pathways with chloroformates to yield N-dealkylated pyrrolidines and 4-chlorobutyl carbamates. The pathway taken depends on the substituent on the nitrogen, i.e., ring-opening with methyl and ethyl substituents and dealkylation with a benzyl substituent. Computational calculations support the substituent-dependent product formation by showing the energy difference between the transition states of both reaction pathways. Selective ring-opening reactions of N-methyl and N-ethyl pyrrolidine derivatives with chloroformates were utilized to prepare various 4-chlorobutyl carbamate derivatives as valuable 1,4-bifunctional compounds. |
|
|
|
| E-mail |
cndvkdi@naver.com |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS