|
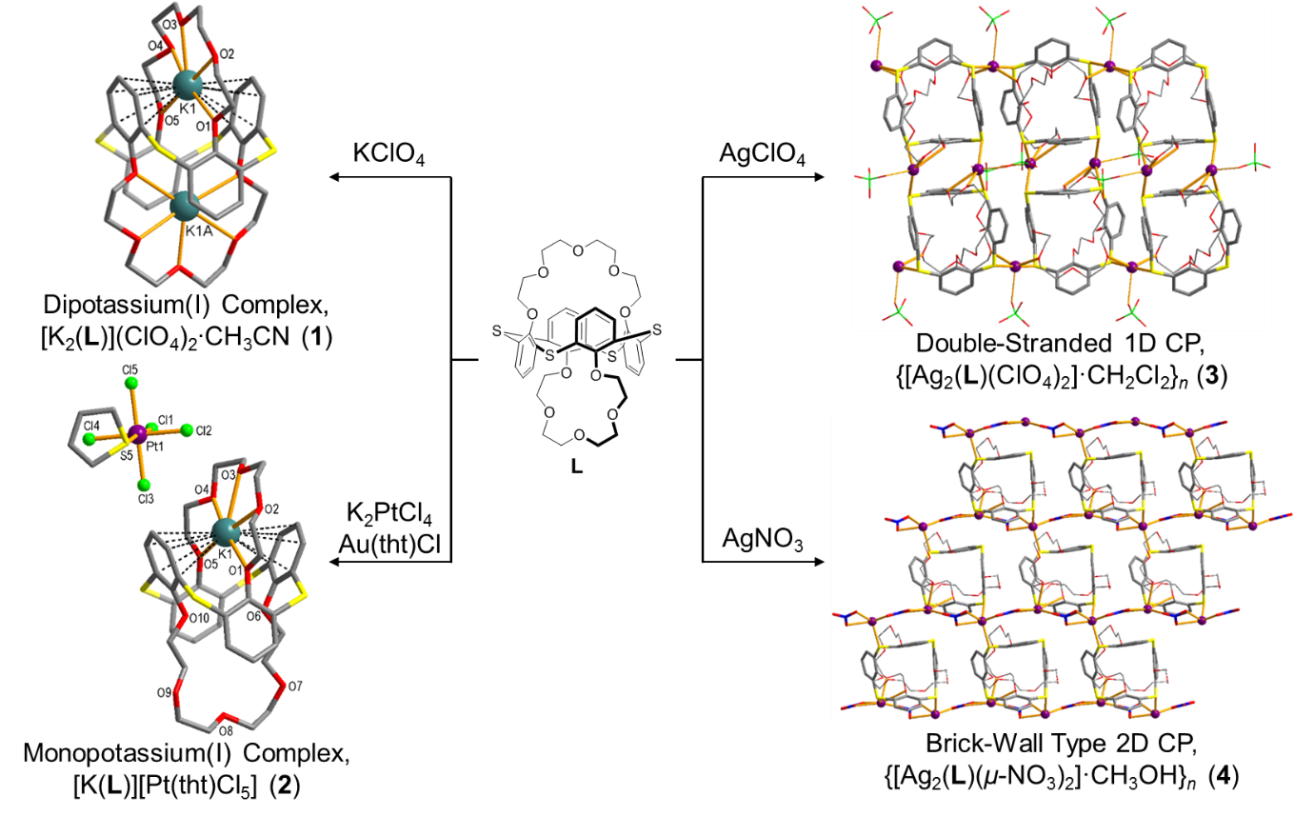
From its potassium(I) and silver(I) complexes, thiacalix[4]-bis-crown-5 (L) with hard crown cavities and soft sulfur-bridges exhibits the characteristics of an HSAB-based complexation and an endo-/exocyclic coordination simultaneously. Reaction of L with KClO4 afforded an endocyclic dipotassium(I) complex [K2(L)](ClO4)2.CH3CN (1). Interestingly, a monopotassium(I) complex [K(L)][Pt(tht)Cl5] (2, tht = tetrahydrothiophene) was isolated from the reaction with a mixed salt as first example of symmetrical calix[4]-bis-crowns. In the complexations with silver(I) salts (3: perchlorate and 4: nitrate), anion-dependent exocyclic coordination polymers with different dimensionalities were isolated. When silver(I) perchlorate was used, for example, a double-stranded 1D coordination polymer {[Ag2(L)(ClO4)2]·CH2Cl2}n (3) was obtained, while silver(I) nitrate afforded a brick-wall type 2D coordination polymer {[Ag2(L)(μ-NO3)2].CH3OH}n (4). Taking dimensionality into account, the topological difference of 3 and 4 is responsible for the different coordination behaviors of the anions: the perchlorato ligand in 3 acts as a terminal, while the nitrato ligand in 4 is a bridging ligand. The solution studies via NMR titrations indicate the endo-coordination with K+ and the exo-coordination with Ag+, similar to those observed in the solid state. |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS