|
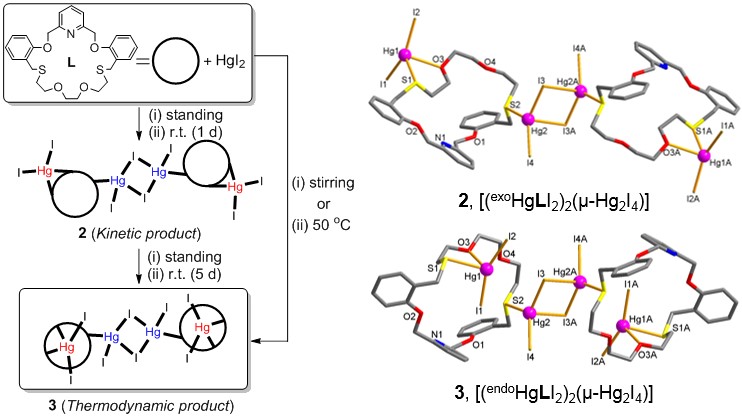
A ditopic 23-membered NO4S2-macrocycle (L) incorporating a rigid and a flexible binding sites was synthesized and its silver(I) and mercury(II) complexes exhibiting different stoichiometries and coordination modes were prepared and structurally characterized. First, silver(I) perchlorate reacts with L to afford an endocyclic mononuclear complex [Ag(L)]ClO4 (1) in which the silver(I) ion locates at the rigid binding site of the macrocyclic cavity adopting a penta-coordinated square pyramidal geometry. The 1H-NMR titration results for the silver(I) complexation agree with the solid state data. Interestingly, reaction of L with HgI2 led to the isolation of two tetranuclear bis(macrocycle) complexes, [(exoHgLI2)2(μ-Hg2I4)] (2) and [(endoHgLI2)2(μ-Hg2I4)] (3), as a kinetic and a thermodynamic product, respectively (see below). These two products are configurational isomers which show different coordination modes. In both products, for example, two exocyclic (2) and two endocyclic (3) mercury(II) complex units are linked by tetraiododimercury(II) core, (μ-Hg2I4), to give a 4:2 (metal-to-ligand) stoichiometry. It is profound that the exo- and endo-coordinated macrocyclic complexes (configurational isomers) are isolated and characterized as a kinetic product and a thermodynamic product, respectively. |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS