|
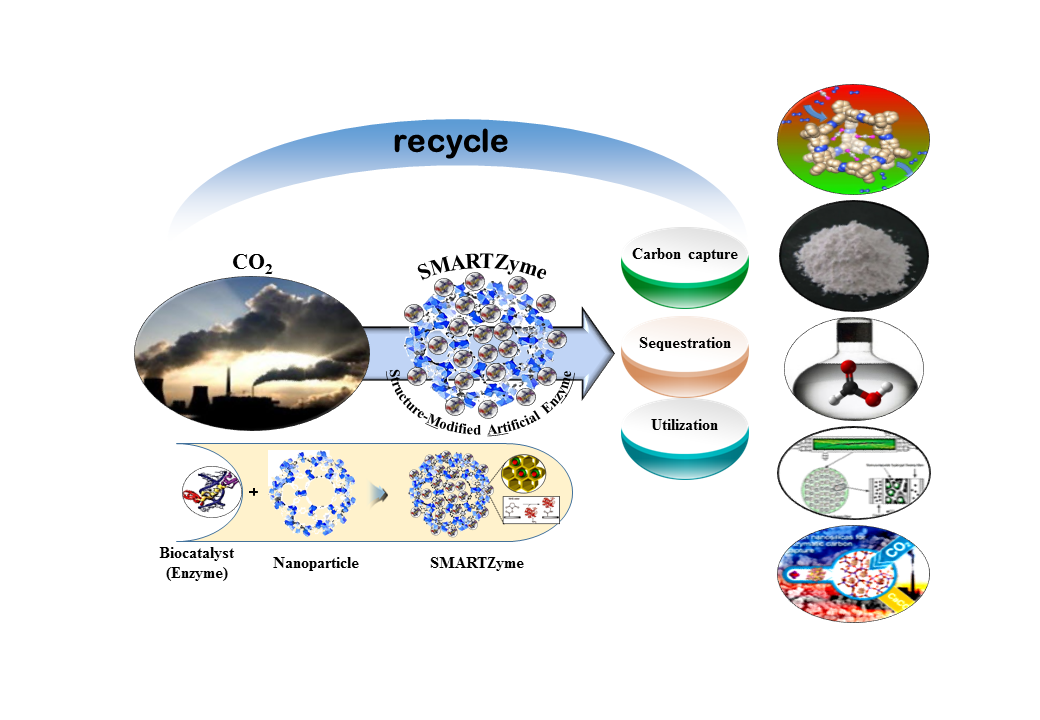
Ultimately, to reduce the concentration of atmospheric CO2, it is necessary to capture, separate, store or reuse CO2. Meanwhile, researchers are working to develop new ways to accelerate CO2 conversion efficiency with value-added compounds using biological, physical, or chemical methods. CO2 conversion to solid form of carbonate appears to be a promising approach. This technology is being studied by several groups around the world and can be used to increase efficiency by using a carbonic anhydrase (CA) enzyme as a catalyst. However, CA enzymes are very expensive, but they are not recyclable and limit their potential in a variety of applications. To solve this problem, the concept of immobilization was utilized. The α-CA gene isolated from Duneliala species was transformed with pET42b vector and purified using a 6 × histidine tag (1.65 mg protein/ml, specific activity 450 U/mg protein). The amount of dissolved CO2 was increased by 3 times by CA treatment at 5 ppm and the amount of calcium carbonate (CaCO3) increased linearly as CaCl2 increased to 100 mM. In order to immobilize the enzyme, the aluminumoxide carrier (Al2O3) surface was functionalized with methanol, octadecyl- trichlorosilane, 1H,1H,2H,2H-perfluoroctyltriethoxysilane, 3-aminopropyltriethoxysilane, 3-mercaptopropyltriethoxysilane, and 3-glycidyloxypropyltriethoxysilane (FT-IR analysis). Of these, more than 99% of the enzyme was fixed and repeated up to 40 times, resulting in an effect of 80% or more. CA has an unusually large maximum turnover rate of 106 mol[CO2]mol-1[CA]sec-1 and therefore has tremendous potential for biological sequestration of CO2. |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS