|
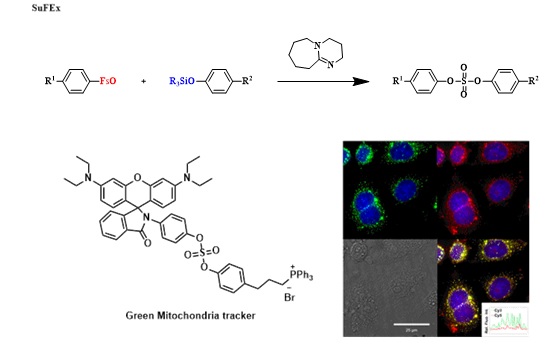
Chemical biology is essential for the study of biological processes in the living system. Especially, site-specific biomolecule functionalization strategies such as spin probes and affinity tags have been greatly utilized for understanding biological system. However, a few drawbacks including side reactions and non-site-specific labeling have been reported. Therefore, bioorthogonal reactions have been devised to tag biological targets within complex living systems. Among those, one of the most powerful methods includes the use of “Click chemistry” such as Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC), strain-promoted alkyne-azide cycloaddition (SPAAC) and tetrazine-trans-cyclooctene ligation. On the other hand, these reactions still have problems in relation with unwanted metal residue, slow reaction rate and chemical instability. Recently, Sharpless et al. discovered a valuable new click reaction for biological chemistry, i.e. sulfur(VI) fluoride exchange (SuFEx) reaction. Herein, we report the effectiveness of the fosylate flurophores as reactive probes in chemical biology and introduce two applications; biorthogonal modification of the side chain of tyrosine derivatives and mitochondria-targeted tracker. |
|
 121st General Meeting of the KCS
121st General Meeting of the KCS
 121st General Meeting of the KCS
121st General Meeting of the KCS