|
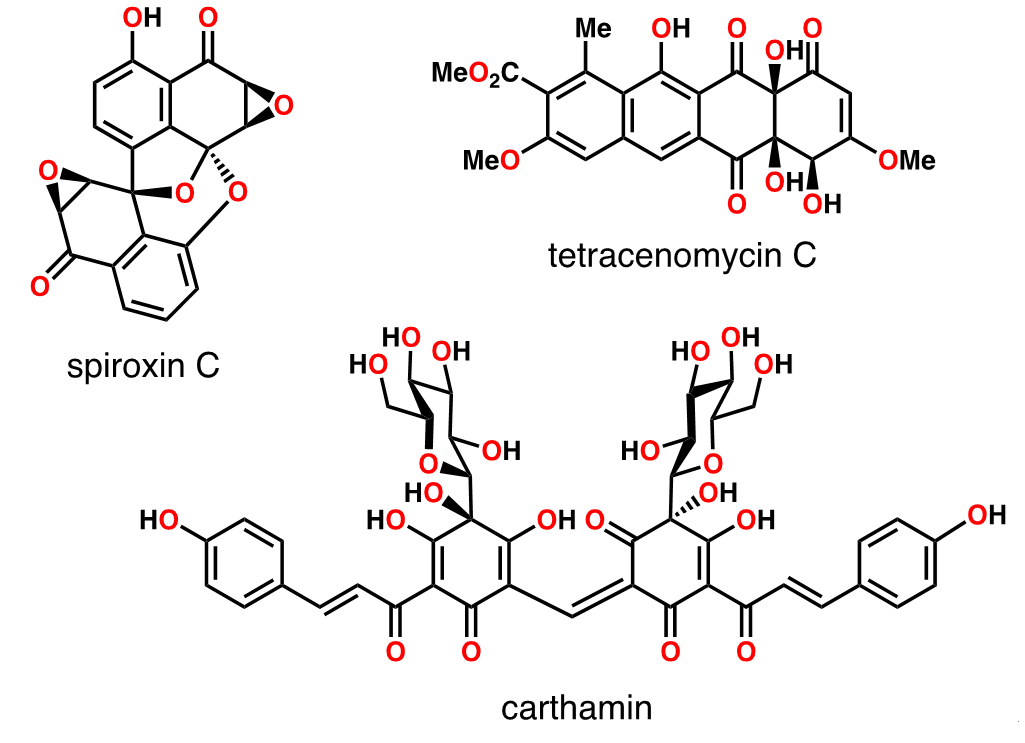
Enchanted by the structural beauty and diversity, we have been engaged in the research on total synthesis of complex natural products. Particularly, we focused attention to the natural products derived from the type-II polyketide biosynthesis, which is as a remarkable pathway, generating an impressive array of compounds characterized by densely functionalized polycyclic architectures with various significant biological activities. The biosynthetic process starts with multiple Claisen condensations of acetate units to generate skipped polyketones, and folding within an enzyme pocket and repeated aldol condensations generate nascent polycycles. Differences in the polyketide chain length as well as the mode of folding provide substantial level of molecular diversity. In addition, the diversity is further enhanced by (1) the post modifications, including oxidative de-aromatization, (2) the dimerization or oligomerization, and (3) the hybridization with other biosynthetic products, such as sugars and/or isoprenoids. This talk will deal with some recent findings made in the synthesis of three natural products.1,2,3,4
References
1) Suzuki, K. Chem. Rec., 2010, 10, 291.
2) (a) Hayashi, D.; Ohmori, K.; Suzuki, K. Synlett, 2016, 27, 2345. (b) Hayashi, D.; Ohmori, K.; Suzuki, K. Org. Lett, 2017, 19, 866.
3) Ando, Y.; Sasaki, Y.; Ohmori, K; Suzuki, K. Angew. Chem. Int. Ed., 2017, 56, 11460.
4) Sato, S.; Sakata, K.; Hashimoto, Y.; Takikawa, H.; Suzuki, K. Angew. Chem. Int. Ed., 2017, 56, 12608.
|
|
 122nd General Meeting of the KCS
122nd General Meeting of the KCS
 122nd General Meeting of the KCS
122nd General Meeting of the KCS