|
N-Containing heterocyclic compounds are extremely important in the study for biological activity and pharmaceutical utilization. Especially, azepinoindoles having both indole and azepine moieties, which are one of the most typical privileged scaffolds, exhibit antifungal properties and function as antilipase, kinase inhibitor, and H1-receptor antagonist.
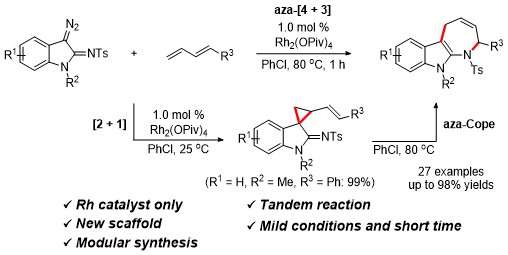
Herein, we developed rhodium-catalyzed formal aza-[4 + 3] cycloaddition reaction of 3-diazoindolin-2-imines with 1,3- dienes for the synthesis of azepinoindoles in good to excellent yields in one-pot. First, rhodium-catalyzed [2 + 1] cycloaddition reaction smoothly took place to produce iminyl vinyl cyclopropane intermediate at room temperature in chlorobenzene for 1 h, which was thermally converted to azepinoindoles through aza-Cope rearrangement.
|
|
 122nd General Meeting of the KCS
122nd General Meeting of the KCS
 122nd General Meeting of the KCS
122nd General Meeting of the KCS