|
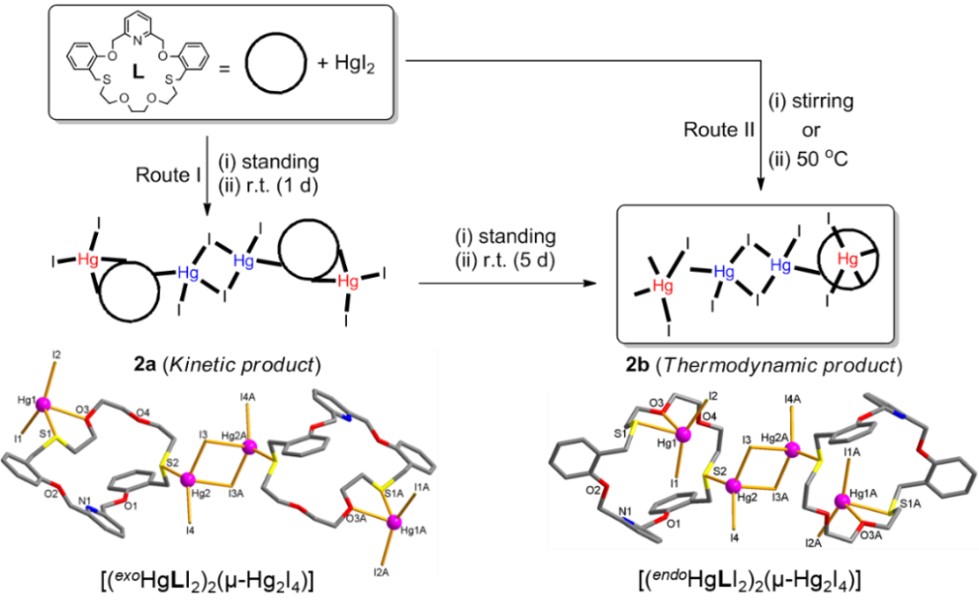
A ditopic 23-membered NO4S2-macrocycle (L) incorporating rigid and flexible binding sites and its soft metal complexes exhibiting different stoichiometries and coordination modes were synthesized and structurally characterized. First, silver(I) perchlorate reacts with L to afford an endocyclic mononuclear complex [Ag(L)]ClO4 (1) in which the silver(I) ion locates at the rigid binding site of the macrocyclic cavity adopting a penta-coordinated SP geometry. NMR titration for the corresponding system also shows of an 1:1 (metal-to-ligand) stoichiometry in solution. Interestingly, reaction of L with HgI2 led to the isolation of two tetranuclear bis(macrocycle) complexes, [(exoHgLI2)2(μ-Hg2I4)] (2a) and [(endoHgLI2)2(μ-Hg2I4)] (2b), as a kinetic and a thermodynamic product, respectively (see below). In both products, two exocyclic (2a) and two endocyclic (2b) mercury(II) complex units are linked by tetraiododimercury(II) core, (μ-Hg2I4), to give a 4:2 stoichiometry. The reaction of L with copper(I) chloride yielded a greenish complex of the formula [CuII(L)Cl2]·CH3CN (3), exhibiting the oxidation of copper(I) to copper(II). When a mixture of CdI2 and HgI2 was used in the reaction with L, a discrete type complex with two separated parts of the formula [Cd(L)I]2[Hg2I6] (4) was isolated. |
|
 122nd General Meeting of the KCS
122nd General Meeting of the KCS
 122nd General Meeting of the KCS
122nd General Meeting of the KCS