|
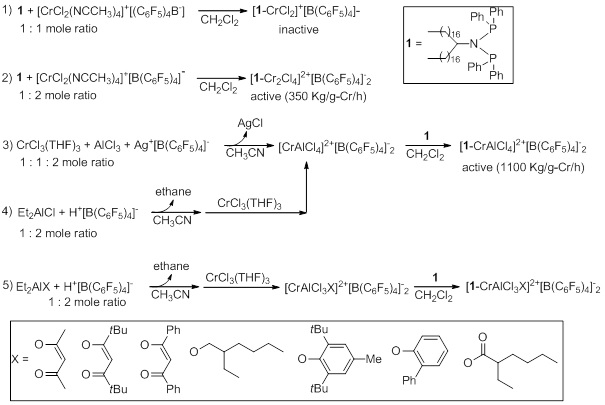
Ethylene tetramerization catalyst systems discovered by Sasol consist of Cr(III) source (Cr(acac)3 or CrCl3), PNP ligand (RN(PPh2)2), and MAO are useful for the production of 1-octene through selective ethylene oligomerization.1 When it comes to the large scale use of the Sasol system, The use of MAO in excess has economic burden because of expensive price of MAO. In this work, we developed a catalytic system using inexpensive alkylaluminum in place of MAO. [(CH3CN)4CrIIICl2]+[B(C6F5)4]- isolated in the reaction of CrCl3(THF)3 with [(CH3CN)4Ag]+[B(C6F5)4]- is reacted with iPrN(PPh2)2 (1) or [CH3(CH2)16]2CHN(PPH2)2 (2) to produce cationic chromiumIII species bearing B(C6F5)4- anion ([{iPrN(PPh2)2}CrCl2(CH3CN)2]+[B(C6F5)4]- or [{[CH3(CH2)16]2CHN(PPh2)2}CrCl2(CH3CN)2]+[B(C6F5)4]-). The molecular structures of [(THF)4CrIIICl2]+[B(C6F5)4]- and [1-CrCl2(THF)2]+[B(C6F5)4]- were unambiguously determined by X-ray crystallography. The cationic (PNP)CrIII complexes paired with [B(C6F5)4]- anions, i.e., [(PNP)CrCl2(CH3CN)2]+[B(C6F5)4]- exhibited high activity with satisfactory selectivity when activated with common trialkylaluminum species (Me3Al, Et3Al, and iBu3Al) in chlorobenzene. Compared to original Sasol system ((iPrN(PPh2)2)CrCl3/MAO (200 Kg/g-Cr/h; 1-hexene 46%, 1-octene 40%, PE 1.2%)), These activity and selectivity are acceptable. When activated with Et3Al or iBu3Al, the Cr complex [2-CrCl2(CH3CN)2]+[B(C6F5)4]- , which bears long alkyl chains, showed high activity in the more desirable methylcyclohexane solvent (89 Kg/g-Cr/h) and much higher activity in cyclohexene (168 Kg/g-Cr/h). Other advantages of the [2-CrCl2(CH3CN)2]+[B(C6F5)4]-/Et3Al system in cyclohexene were negligible amount of side product(polyethylene, 0.3%), generation of fewer unwanted side products above C10, and negligible catalyst deactivation. The [B(C6F5)4]- anion is compatible with trialkylaluminum species once it is not paired with a trityl cation. Therefore, [(PNP)CrCl2(CH3CN)2]+[B(C6F5)4]-/Et3Al exhibited considerably higher activity than that of a previously reported system consist of [Ph3C]+[B(C6F5)4]-, i.e., 1/CrCl3(THF)3/[Ph3C]+[B(C6F5)4]-/Et3Al.
Scheme 1 (or Figure 1).
Reference
[1] M.J. Overett, K. Blann, A. Bollmann, J.T. Dixon, D. Haasbroek, E. Killian, H. Maumela, D.S. McGuinness, D.H. Morgan, Mechanistic Investigations of the Ethylene Tetramerisation Reaction, Journal of the American Chemical Society, 127 (2005) 10723-10730.
|
|
 122nd General Meeting of the KCS
122nd General Meeting of the KCS
 122nd General Meeting of the KCS
122nd General Meeting of the KCS