|
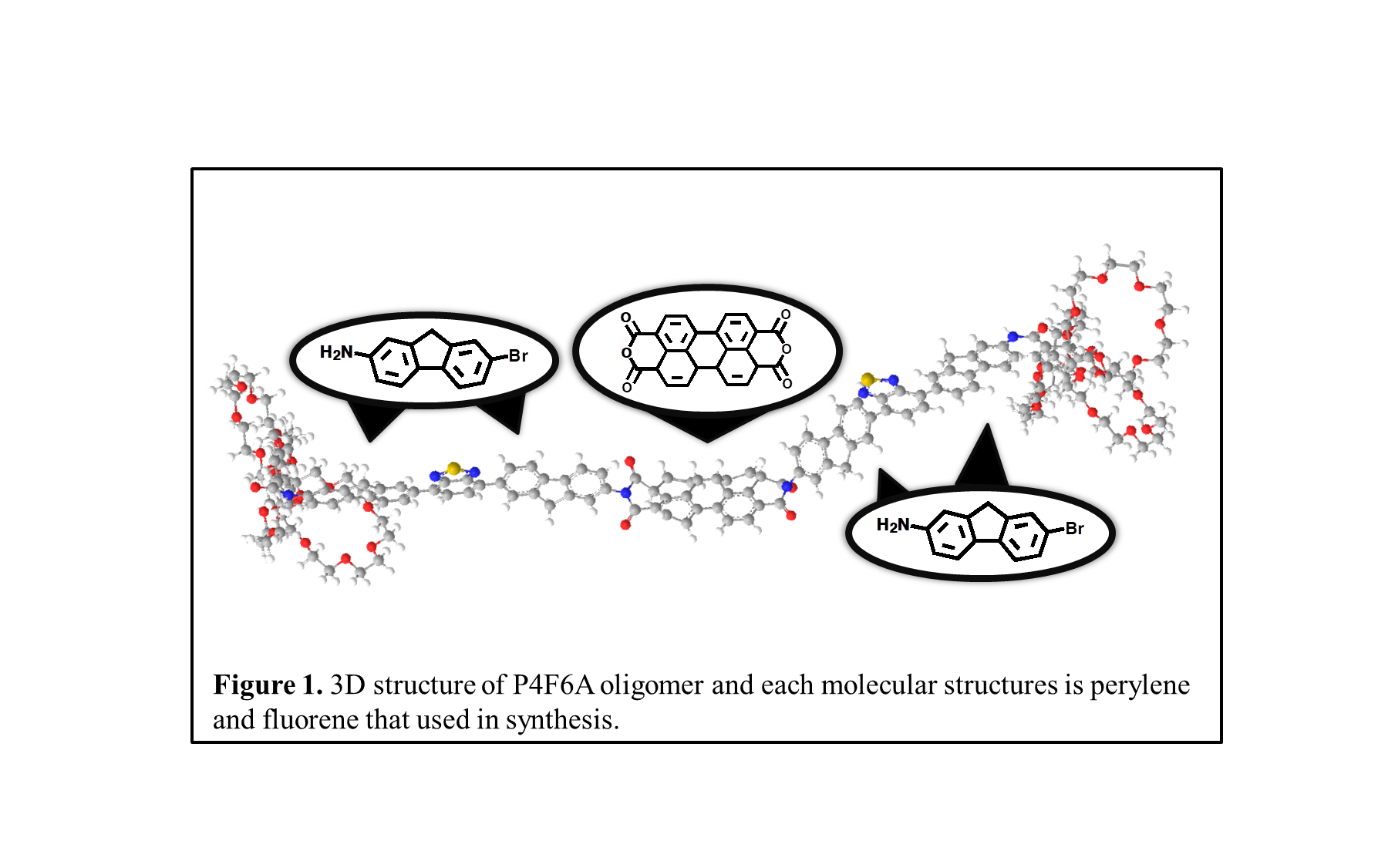
For the research of organic semiconductors, we focused on Donor-acceptor oligomers. That is composed of four 2-Amino-7-bromofluorene (fluorene) as the electron donor and one perylene-3,4,9,10-tetra carboxylic dianhydride (perylene) as the electron acceptor. Final product can be used to fabricate a one-dimensional fibrillated optoelectronic device. Typically, Perylene bisimides (PBI) belong to a class of n-type chromophores exhibiting relatively high electron mobility, large molar absorption coefficients, fluorescence quantum yield, as well as good thermal and photochemical stabilities. Two fluorene and benzothiazole are synthesized by Suzuki coupling. A long chain is formed by attaching 6 alkyl chains on one side of flourene, and it can be purified by chromatography. The long chain thus synthesized is a chromophore, which is attached to both side of perylene to synthesize N,N'-((7,7'-((1,3,8,10-tetraoxo-3,3a,7a,8-tetrahydroanthra[2,1,9-def:6,5,10-d'e'f']diisoquinoline-2,9(1H,5aH,5bH,10H)-diyl)bis(9H-fluorene-7,2-diyl))bis(benzo[c][1,2,5]thiadiazole-7,4-diyl))bis(9H-fluorene-7,2-diyl))bis(3,4,5-tris(2,5,8,11,14,17-hexaoxanonadecan-19-yloxy)benzamide) (P4F6A). Thus, the products allow for conjugation over a wider wavelength range. It is possible to conform π- π stackings of perylene and fluorene, that have more photochemical stability. Synthesized oligomers can be confirmed by NMR spectroscopy and MALDI-TOF. And, The photochemical properties can be confirmed by UV spectroscopy. |
|
 122nd General Meeting of the KCS
122nd General Meeting of the KCS
 122nd General Meeting of the KCS
122nd General Meeting of the KCS