|
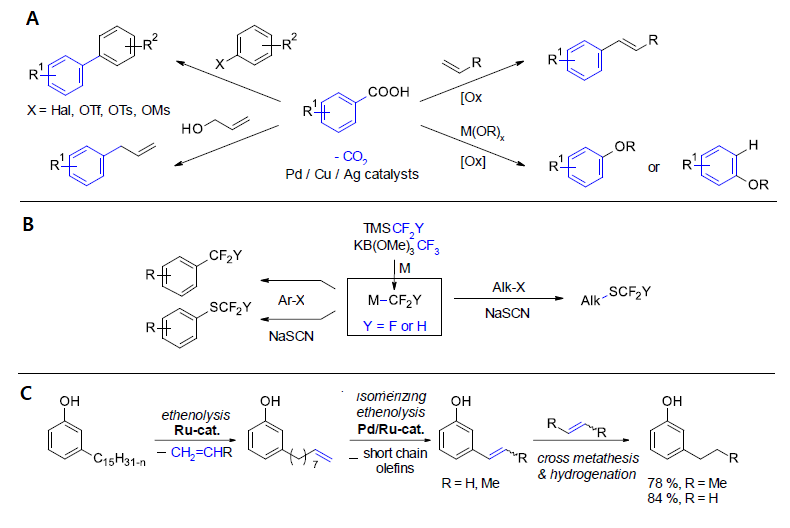
Since our initial report in 2006, decarboxylative coupling reactions, i.e. reactions in which C–C bonds to carboxylate groups are cleaved with formation of new carbon–carbon bonds, have evolved into a powerful synthetic strategy. Their key benefit is that they draw on easily available carboxylic acids rather than expensive organometallic reagents as sources of carbon nucleophiles. Decarboxylative couplings have been utilized e.g. in syntheses of biaryls, vinyl arenes, aryl ketones and aryl ethers. The decarboxylative Chan-Evans-Lam alkoxylation of benzoic acids demonstrates that this reaction concept is applicable also to C–heteroatom bond-forming reactions.
In recent variations of this reaction type, the carboxylate groups are first utilized as directing groups for ortho-C–H functionalizations and then either cleaved tracelessly or used as leaving groups in subsequent ipso-substitution reactions. In such transformations, the arene substitution pattern of the benzoate substrates is altered in a defined way, so that they ideally complement the preceding protocols.
Besides decarboxylative couplings, other sustainable C–C and C–heteratom bond-forming concepts will be
discussed including fluoroalkylations and isomerizing olefin metatheses.
References
(a) L. J. Gooßen, G. Deng, L. M. Levy, Science 2006, 313, 662–664. (b) S. Bhadra, W. I. Dzik, L. J. Gooßen, Angew. Chem. Int. Ed. 2013, 52, 2959–2962. (c) B. Bayarmagnai, C. Matheis, K. Jouvin, L. J. Gooßen, Angew. Chem. 2015, 127, 5845-5848. (d) L. Huang, A. Biafora, G. Zhang, V. Bragoni, L. J. Gooßen, Angew. Chem. Int. Ed. 2016, 55, 6933–6937. (e) K. F. Pfister, S. Bader, M. Baader, S. Berndt, L. J. Goossen, Sci. Adv. 2017, 3, e1602624. (f) X.-Q. Hu, Z. Hu, A. S. Trita, G. Zhang, L. J. Gooßen, Chem. Sci. 2018, 9, 5289–5294. |
|
 122nd General Meeting of the KCS
122nd General Meeting of the KCS
 122nd General Meeting of the KCS
122nd General Meeting of the KCS