|
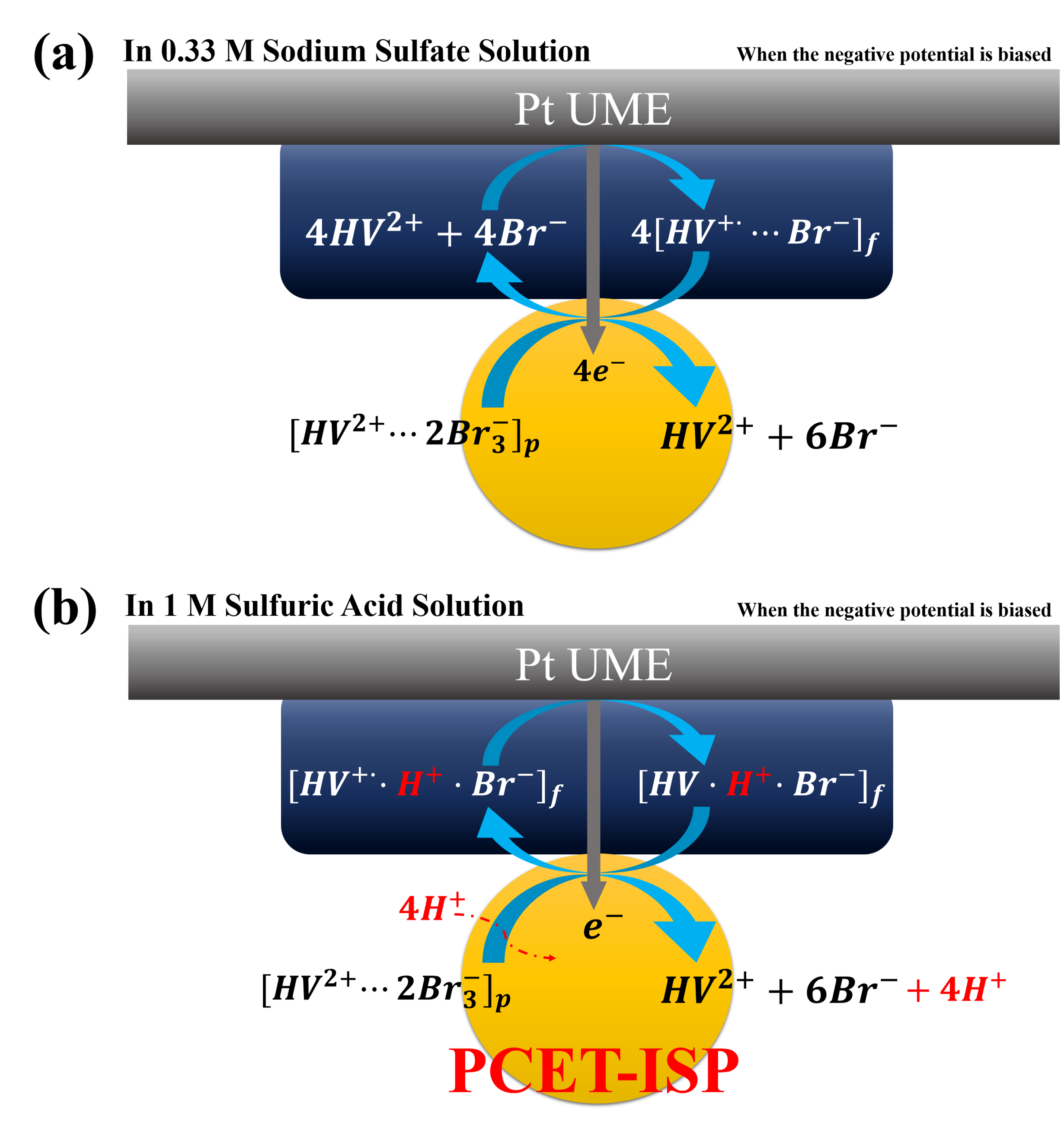
Here we present the electrochemistry based on the formation/dissolution reactions of two dual-RICs(Redox ionic complexes) : HV2+ and Br-. During a charge process, HV2+ is reduced to form a solid [HV+··Br-] on an anode, while Br- is oxidized to from a solid [HV2+·2Br3-] on a cathode, resulting in significant suppression of self-discharge in the Redox-EC(electrochemical capacitors). We found that [HV+··Br-](s) formed on Pt UME is electrochemically further reduced through proton coupled electron transfers (PCETs). Also, we deciphered stochastic current spikes associated with the galvanic exchange reaction between a [HV+··Br-](s) film and a [HV2+·2Br3-] particle in an acidic and neutral solution, and proposed the different electro-reduction mechanism of [HV2+·2Br3-] particles on a modified Pt UME with [HV+··Br-] in acidic condition from that in a solution with neutral pH. |
|
 123rd General Meeting of the KCS
123rd General Meeting of the KCS
 123rd General Meeting of the KCS
123rd General Meeting of the KCS