|
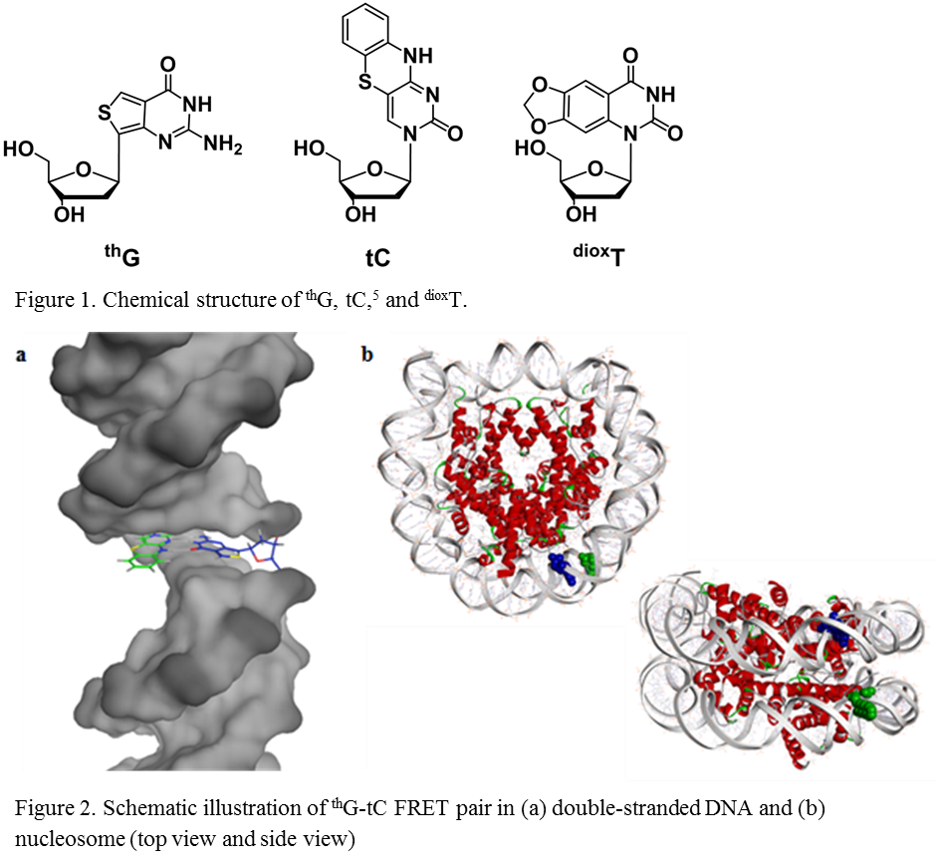
Fluorescent nucleoside analogues have received much attention as powerful tools for chemical biology. Their applications have expanded our understanding of biological phenomena by visualizing DNA. Previously, we have developed fluorescent guanosine analogue, thG. It can be readily applied in solid-phase synthesis; moreover, its triphosphate was enzymatically incorporated into DNA through PCR amplification. We visualized structural change of DNA from right-handed B-form to left-handed Z-form using its highly emissive property.1, 2 Furthermore, we developed new FRET system using thG as a donor and tC as an acceptor. In this system, FRET efficiency depends on both distance and orientation factor because the fluorophores are strictly fixed in double helix structure. We introduced this system into nucleosomal DNA and demonstrated that isomorphic nucleobase FRET system could be useful for studying nucleosome structure and its dynamics.3, 4 Very recently, we have synthesized a new fluorescent thymidine analogue, dioxT. It exhibits high affinity with adenine as thymine surrogate and relatively high quantum yield in DNA duplex. Now, we are studying the construction of FRET system which consists of dioxT and tC. In this presentation, I introduce our research on fluorescent nucleosides.
1) Park, S. et al. Chem. Commun. 2014, 50, 1573.
2) Otomo, H. et al. RSC Adv. 2014, 4, 31341.
3) Han, J. H. et al. Chem. Eur. J. 2017, 23, 7607.
4) Han, J. H. et al. Chem. Eur. J. 2018, 27, 17091.
5) Wilhelmsson, L. M. et al. J. Phys. Chem. B. 2003, 107, 9094.
|
|
 123rd General Meeting of the KCS
123rd General Meeting of the KCS
 123rd General Meeting of the KCS
123rd General Meeting of the KCS