|
Far-red/NIR emitting organic fluorophores are in great demand for microscopic tissue and whole-body imaging with minimal autofluorescence and reduced light scattering. Currently, only a few classes of far-red/NIR fluorophores are available. Herein, we present novel classes of far-red/NIR-emitting dyes that are photo-stable, very bright, biocompatible, and also two-photon absorbing.
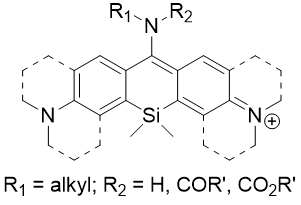
Introduction of an electron-withdrawing group on the C-10-amino substituent of amino-Si-pyronin dyes (aASiP) caused huge bathochromic shifts, leading to the NIR-emitting amino-Si-pyronin dyes (NIR-aASiP). However, the NIR-aASiP dyes showed very dim cellular images in the far-red/NIR wavelength region but strong emission in the shorter wavelength (yellow) region. The unexpected cellular imaging results indicated chemical instability inside cells, which is plausibly caused by nucleophilic attack of biomolecules (biothiols or amines). This biostability issue was solved by converting acyclic amine donor into julolidine-derived donor that increases the electron density at C-10 and thus protects it from nucleophilic attack by the biomolecules. The julolidine-derived NIR-emitting amino-Si-pyronin dyes (NIR-jASiP) have high cellular stability as well as two-photon absrobing property. The far-red and NIR emitting amino-Si-pyronin dyes constitute a promising fluorescence resonance-energy transfer (FRET) system and both dyes have great potential for biomedical imaging application
|
|
 123rd General Meeting of the KCS
123rd General Meeting of the KCS
 123rd General Meeting of the KCS
123rd General Meeting of the KCS