|
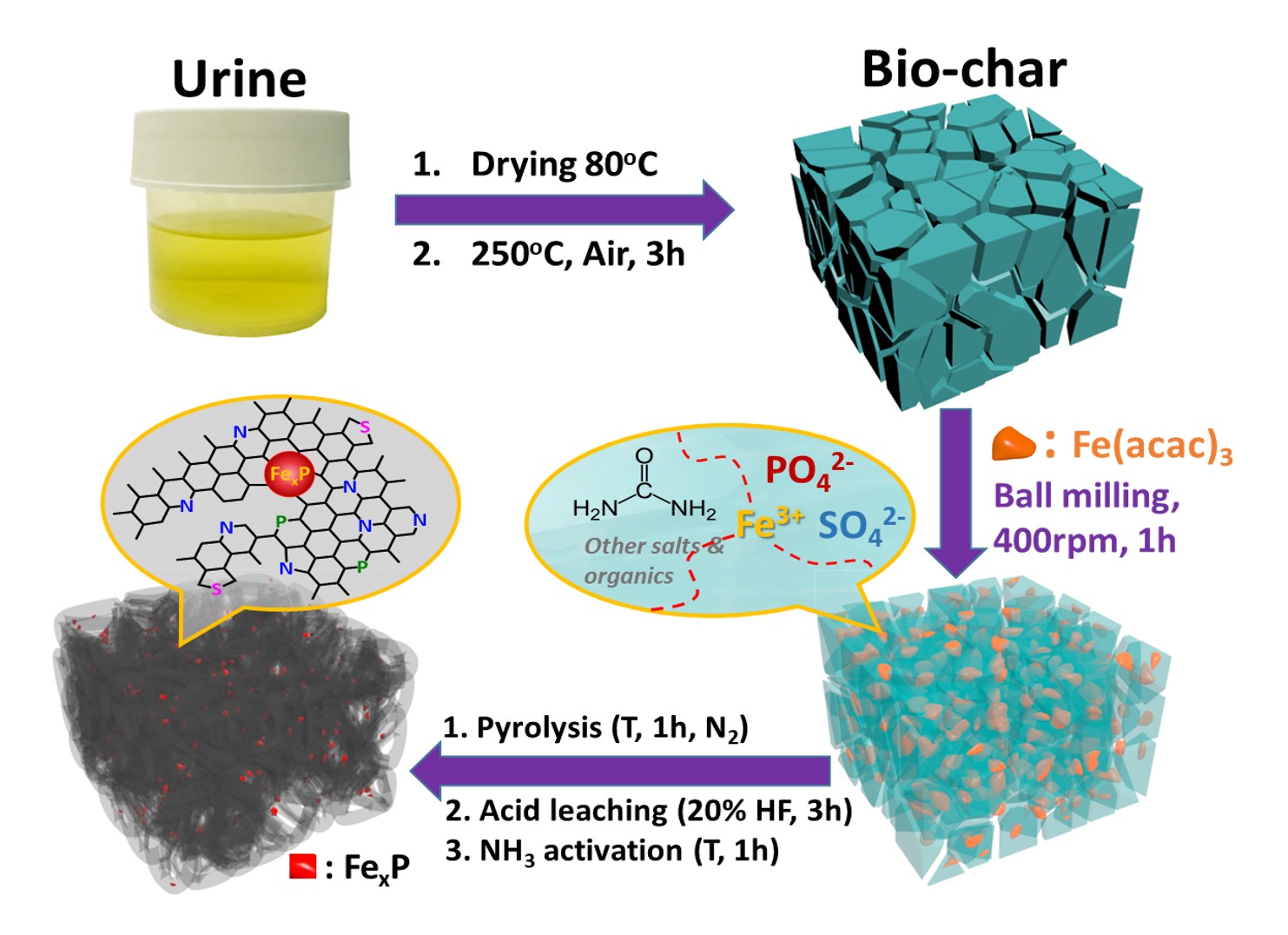
The development of electrocatalysts from inexpensive, natural sources has been an attractive subject owing to economic, environmental, sustainable, and social merits. [1] Herein, Fe‐treated heteroatoms (N, P, and S)‐doped porous carbons are synthesized for the first time by pyrolysis of bio‐char derived from abundant human urine waste as a single precursor for carbon and heteroatoms, using iron(III) acetylacetonate as an external Fe precursor, followed by acid leaching and activation with a second pyrolysis step in NH3. In particular, the sample prepared at a pyrolysis temperature of 800 °C (FeP‐NSC‐800) contains iron phosphide (FeP, Fe2P)[2] in the high‐porosity heteroatoms‐doped carbon framework along with Fe traces, and exhibits excellent oxygen reduction reaction (ORR) activity and stability in both alkaline and acidic electrolytes as demonstrated in half‐ and single‐cell tests. Such excellent ORR catalytic performance is ascribed to a synergistic effect of multiple active Fe−P, Fe−N, and pyridinic and graphitic N species in the electrocatalyst as well as facile transport channels provided by its hierarchical porous structure with micro‐/mesopores. In addition, the sample exhibits high long‐term durability and methanol crossover resistance.
References
[1] T.-N. Tran, M.Y. Song, K.P. Singh, D.-S. Yang, J.-S. Yu, J. Mater. Chem. A 2016, 4, 8645.
[2] K.P. Singh, E.J. Bae, J.-S. Yu, J. Am. Chem. Soc. 2015, 137, 3165.
Figure 1. Schematic illustration of the preparation process for FeP‐NSC‐T electrocatalysts |
|
 123rd General Meeting of the KCS
123rd General Meeting of the KCS
 123rd General Meeting of the KCS
123rd General Meeting of the KCS