|
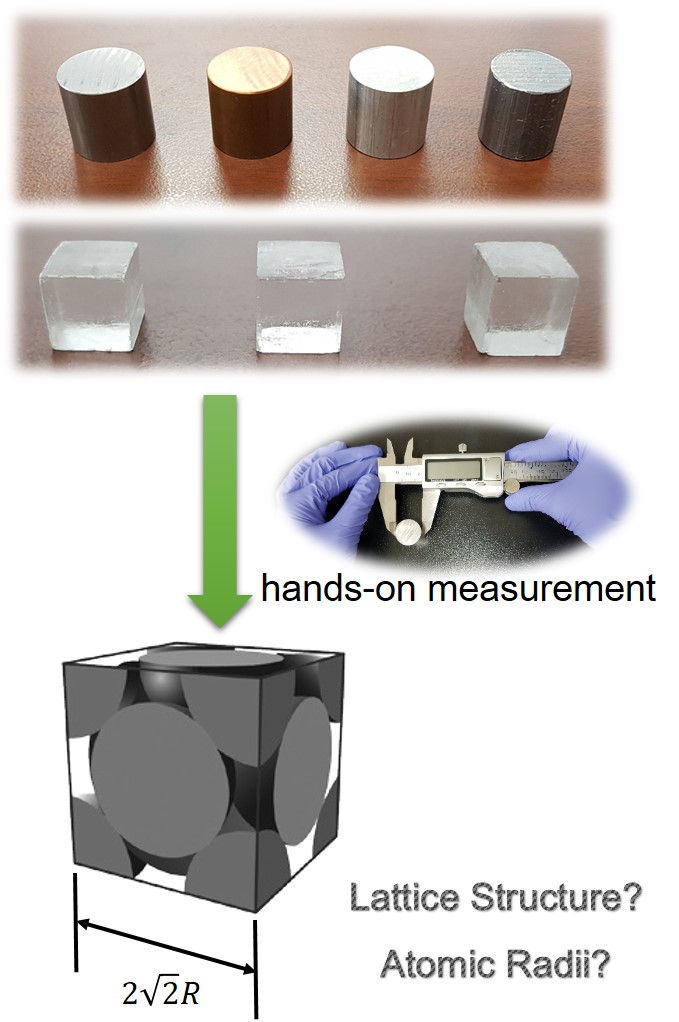
A hands-on experiment to obtain atomic and ionic radii with lumps of metal and ionic compounds is reported here. The experiment is performed with industrial-grade typical metals (iron, copper, aluminum, and lead) and single-crystal lumps of ionic compounds (sodium chloride, potassium chloride, and potassium bromide). Students measure the dimension of the given lumps with Vernier calipers and weigh them with a precision balance. After measuring the dimension and mass of the given lumps, students can calculate the atomic and ionic radius of each corresponding atom and ion with the obtained density values and information about the lattice structures. The obtained values of the metal atomic radii and the ionic radii of ionic compounds are very close to values in the literature. After this activity, the students came to appreciate that atomic radii, as typical sub-microscopic and symbolic representations, are actually existing features and not an abstract concept. The experiment reported here is suitable for a first-year undergraduate general chemistry class as well as an introductory class at the high school level. |
|
 123rd General Meeting of the KCS
123rd General Meeting of the KCS
 123rd General Meeting of the KCS
123rd General Meeting of the KCS