|
We have previously reported the synthesis of ene-hydrazide from enol triflate and subsequent indolization reaction as a new entry to a regioselective Fischer indole synthesis.1 In this process, a base-catalyzed intramolecular aza-Michael reaction, in situ trapping of the resulting enolate, and subsequent C-N coupling with phenyl hydrazide afforded the key ene-hydrazide. This new synthetic strategy has been successfully applied to the total synthesis of (+)-aspidospermidine and (-)-tabersonine.2
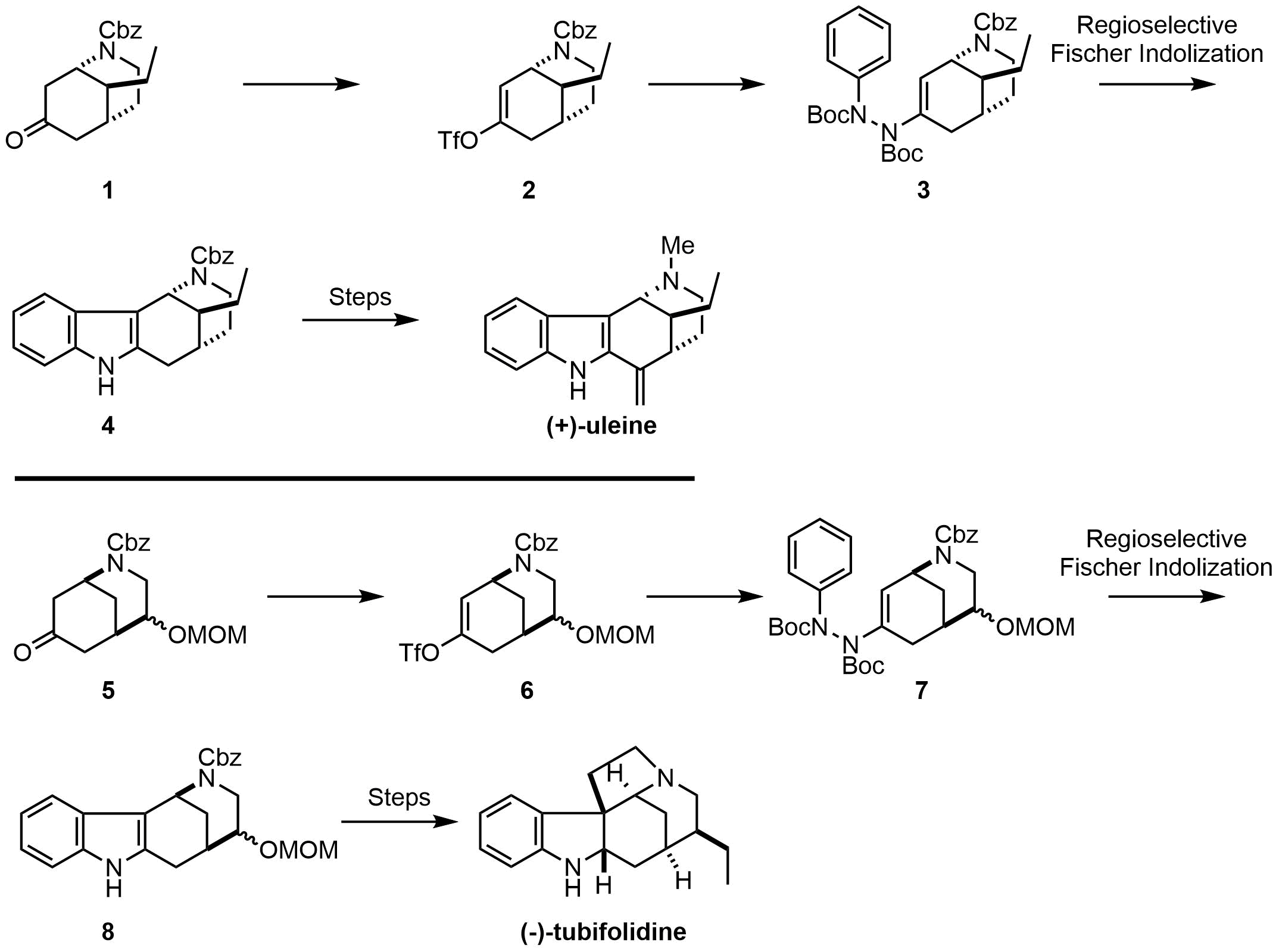
Toward further development of our strategy, we have envisaged a new synthetic route to (+)-uleine and (-)-tubifolidine by ways of bicyclic carbamates 1 and 5. Formation of enol triflates 2 and 6 followed by C-N coupling reactions with phenyl hydrazide and regioselective Fischer indolization under Lewis acidic conditions would selectively give desired indole 4 and 8, respectively. Our recent progress on the total syntheses of (+)-uleine and (-)-tubifolidine will be presented.
References
1. Lim, B.-Y,; Jung, B.-E,; Cho, C.-G. Org. Lett. 2014, 16, 4492-4495.
2. Kim, J.-Y.; Suhl, C.-H.; Lee, J.-H.; Cho, C.-G. Org. Lett. 2017, 19, 6168-6171.
|
|
 123rd General Meeting of the KCS
123rd General Meeting of the KCS
 123rd General Meeting of the KCS
123rd General Meeting of the KCS