|
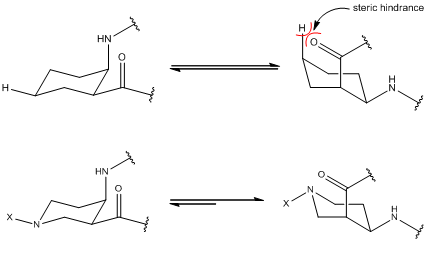
Generally, we can study various helical secondary structures for synthesis of many peptide oligomers containing various residues. This research focus on α/β-peptides oligomers containing 6-membered ring β-residues because , from the previous study, we researched that α/β-peptides oligomers containing (1R,2S)-2-Aminocyclohexanecarboxylic Acid (cis-ACHC) and L-Ala have two intramolecular hydrogen bonds; C=O(i) ∙∙∙ H-N(i+3) and C=O(i) ∙∙∙ H-N(i-1). But in this molecule structure, we thought that axial carbonyl group at C4 in ACHC has steric hinderance because of the axial hydrogen at C6 in ACHC. Therefore using (3R, 4R)-4-Aminopiperidine-3-carboxilic acid(cis-APiC), we remove steric hinderance and expect that α/β-peptides oligomer`s helix property is raised. For this research, we study with CD, IR, 2D NMR data and single crystal structure and compare with on α/β-peptides oligomers containing ACHC and Cis-AiPC protected Tosyl group in N6. |
|
 123rd General Meeting of the KCS
123rd General Meeting of the KCS
 123rd General Meeting of the KCS
123rd General Meeting of the KCS