|
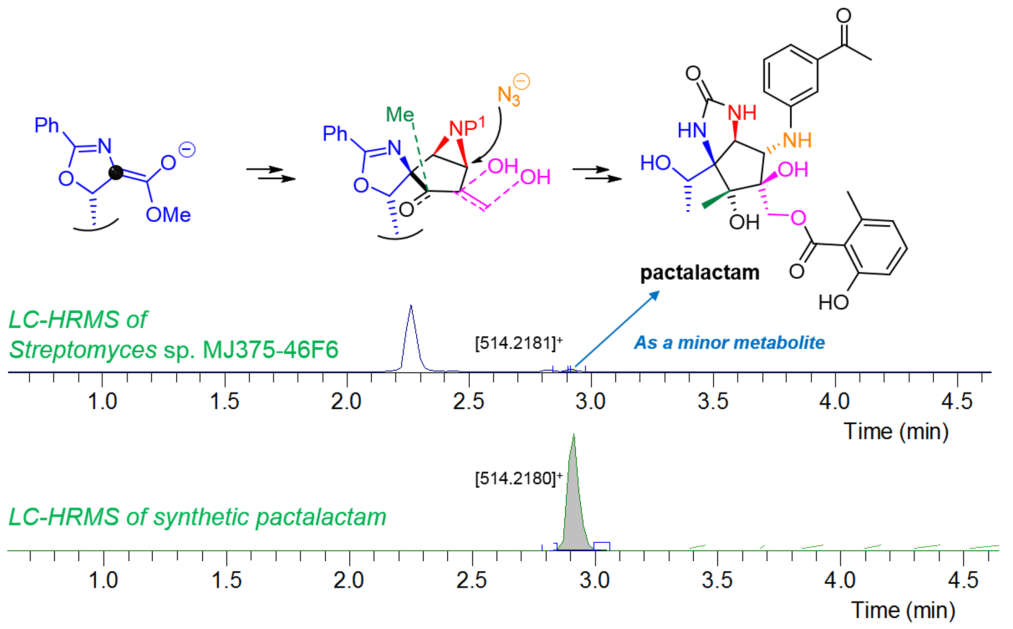
Pactalactam, with a densely functionalized aminocyclopentitol core featuring six contiguous stereogenic centers, was isolated from a fermentation broth of Streptomyces pactum var. pactum as a minor component in 1980. Pactamycin, a major component of the above culture broth, has a wide range of biologically attractive properties and a unique chemical structure. However, its development as a clinical drug was curtailed due to its broad and potent cytotoxicity. Therefore, the synthesis of pactalactam a cyclic urea pactamycin analog, has focused on the development of new synthetic strategy, and securing biological research data and chemical information. The cyclopentane framework was obtained by means of ring-closing metathesis (RCM) and aldol coupling with L-threonine-derived optically active oxazoline. The key steps were the substrate-controlled stereoselective aziridination and the regioselective aziridine ring-opening by azide for the construction of three-continuous amino groups, face-selective dihydroxylation, and addition of methyl anion on the octa-substituted cyclopentane core. Finally, reductive oxazoline ring-opening reaction, construction of the cyclic urea moiety, 3-acetylphenyl group introduction by Cu-catalyzed C-N bond formation, primary alcohol-selective acylation and sequential deprotection gave reported structure of pactalactam. Furthermore, the existence of pactalactam in culture broth of pactamycin-producing Streptomyces sp. was revealed by comparison of synthetic sample. |
|
 123rd General Meeting of the KCS
123rd General Meeting of the KCS
 123rd General Meeting of the KCS
123rd General Meeting of the KCS